Positive Health Online
Your Country

Stress and Ageing: The Micro-Immunotherapy Approach
listed in immune function, originally published in issue 232 - August 2016
I. Introduction
Stress has considerable effects on the immune function. Recent studies have suggested that the aging of the immune system (or immunosenescence) is, in part, closely linked to psychological stress factors and stress hormones. Chronic stress leads to premature ageing of the essential homeostatic systems, which are central to the adaptation of organisms to environmental changes.[1]
In young individuals, effective immune responses can compensate for the effects of stress. However, as we age, the immune system deteriorates, due to both the ageing of the various components of the immune system and the establishment of a chronic pro-inflammatory state known as “inflammaging”.[2]
The characteristics of immuno-senescence (whether natural or induced by stress) include:
- High levels of oxidative stress (reactive oxygen species; ROS);
- Persistent chronic inflammation;
- Telomere shortening and telomerase inactivation;
- Chronic exposure to endogenous glucocorticoids;
- Decrease in cellular immunity.
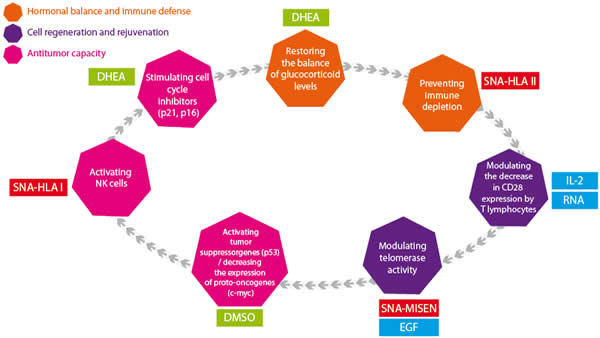
Description of the components, objectives and sequential cascade of the treatment
Specific nucleic acid SNA®-HLA II
The Micro-Immunotherapy MISEN formula has a composition and sequential nature rendering it highly suitable for regulating and stabilizing parameters altered by stress and the aging process.
Mechanism of Action
- The MISEN formula alternates sequences for stimulating regeneration, proliferation and effective functioning in diverse cell lines with sequences designed to optimize apoptosis, cell cycle inhibition and the expression of tumour suppressor genes;
- The objective is to promote cellular rejuvenation without increasing the risk of neoplastic proliferation;
- The MISEN formula, thus, aims to equilibrate the factors leading to cellular ageing, such as chronic exposure to endogenous glucocorticoids, thereby compensating for deficiencies resulting from the process of senescence.
II. Mode of Action of the MISEN Formula
Composition
Dilutions: Stimulating - Modulating - Inhibitory
Senescence is a process that generally occurs in response to stress and DNA lesions. This natural response to DNA damage protects the body against cancer,[3] but leads to a large increase in the levels of ROS and other pro-inflammatory factors associated with cell death (or apoptosis), which in turn contributes to tissue ageing. During senescence, inflammation may be observed, due to the secretion of age-linked inflammatory factors (the senescence-associated secretory phenotype or SASP), which aggravates the imbalance. This results in a persistent, chronic inflammation of various degrees of severity.[4]
The triggering, propagation and modulation of the adaptive immune response depend on the precise regulation of MHC class II molecules. Unlike MHC class I molecules, which are widely expressed in different cell types, the expression of MHC class II molecules, such as HLA-DR, is restricted to antigen-presenting cells (professional APCs) including macrophages, dendritic cells and B lymphocytes. By contrast, in inflammatory conditions,[5] such as those occurring during immuno-senescence, HLA-DR expression may be triggered in cells that did not initially present antigens (non-professional APCs), such as epithelial cells and keratinocytes.[6,7,8]
The ‘repeated’ presentation of antigens to T lymphocytes by non-professional APCs is one of the causes of a loss of T-cell effector function, which manifests as a loss of CD28 expression by these cells, particularly in a context of chronic inflammation.[9,10] CD28 is an essential co-stimulation receptor responsible for the activation, proliferation and survival of T cells.[11] CD28 co-stimulation is also necessary for the effective activation of CD4+ T lymphocytes, because it plays an essential role in the establishment of effector function in these cells.[12]
Immune-Modulating Cells
SNA®-HLA II
Objective:
To inhibit the overexpression of MHC class II molecules in non-professional APCs in inflammatory environments, so as to decrease the immune depletion of CD8+ T lymphocytes and its consequences.
Interleukin 2 (IL-2)
The accumulation of CD28- T lymphocytes, particularly within the CD8+ subpopulation, is one of the most important changes associated with ageing. CD28 co-stimulation favours T-lymphocyte immune responses and the expansion of T-cell populations, by stimulating the production and secretion of IL-2.[13,14]
Immuno-senescence is characterized by specific re-modelling of the immune system, triggered by chronic exposure to antigens and oxidative stress. As the immune system ages, adaptive immunity deteriorates, due to gradual decreases in the numbers of naïve T and B lymphocytes and absolute numbers of T and B lymphocytes.[15] The telomeres play an important role in this process.
Telomeres are specialized structures located at the ends of chromosomes; they gradually shorten over successive cell divisions; this shortening is inversely proportional to age.[16] They protect the ends of the chromosomes from DNA degradation and repair activities. Telomerase is required for telomere repair.[17]
Excessive shortening of the telomeres leads to cell cycle arrest or replicative senescence. The immune system is very sensitive to telomere shortening, because its efficacy is entirely dependent on cellular renewal and the expansion of T and B lymphocyte populations. Immune cells (CD4+ T lymphocytes, CD8+ T lymphocytes, B lymphocytes, granulocytes, monocytes and NK cells) are the only cells capable of increasing telomerase activity, thereby favouring a lengthening of telomeres and limiting their degradation in activated cells.[18]
Interleukin 2 is an essential immuno-regulatory cytokine, because it increases telomerase activity in immune cells, such as lymphocytes and NK cells.[19,20]
IL-2
Objective:
To attenuate the consequences of the continual decrease in CD28 expression by T lymphocytes and to stimulate telomerase activity in immune cells.
Ribonucleic acid (RNA)
Type I interferons (IFN-α and IFN-β) are a family of pro-inflammatory cytokines essential for antiviral immunity.[21] However, their overproduction is associated with various autoimmune diseases.[22] IFN-α is secreted in response to viral RNA during viral infections.
The activity of type I IFNs in the presence of low-level inflammation or a pro-inflammatory environment linked to senescence[2] favours the production of CD28- cells. For this reason, during ageing, persistent viral infections, episodes of viral reactivation or repeated infections provoking the continuous activation of the TCR (the T-cell antigen receptor) and, thus, increasing the secretion of IFN-α, favour the accumulation of T cells with a senescent CD8+ CD28- phenotype.[23]
IFN-α inhibits telomerase activity, thereby accelerating the differentiation of CD8+ T lymphocytes.[23] In addition to its effects on T lymphocytes,[24] this cytokine also inhibits telomerase activity in cells of other lineages, such as hematopoietic cells,[25,26] by diverse mechanisms.
The stimulation of Toll-like receptors (TLR) and their signalling pathways triggers the secretion of type I IFNs. The TLR7, TLR8 and TLR9 receptors in plasmacytoid dendritic cells (pDCs) trigger the production of IFN-α in response to viruses and the binding of their ligands.[27,28,29,30]
Synthetic and viral single-stranded RNA molecules are the ligands of the TLR7 and TLR8 receptors.[31,32,33]
RNA
Objective:
To modulate the secretion of type I IFNs and their negative effects on CD28 expression and telomerase activity, without losing the defensive capacities provided by these molecules.
Specific Nucleic Acid SNA®-MISEN
Their genes, the environment and chance factors influence the longevity of animals.[34] All genetic mutations affecting endocrine pathway signaling, stress responses, metabolism or telomere length may influence the longevity of organisms.[35]
Diverse factors regulating gene expression can modify the ageing process, by modulating tissue deterioration or cell senescence.[36]
SNA®-MISEN
Objective:
To inhibit genes with stress- or ageing-specific expression.
Epidermal Growth Factor (EGF)
Cell senescence is a safety response, protecting against cancerous transformation.[37] Senescence occurs when cells display a critical shortening of telomeres, due to DNA replication mechanisms. Gradual shortening of the telomeres, in the absence of telomerase, has no effect on the cell cycle. By contrast ‘critical’ telomere shortening plays a key role in senescence.[38] Telomere shortening is one of the fundamental mechanisms of ageing and of lifespan limitation.
Telomerase is a highly regulated enzyme: its activity is closely associated with cell proliferation. In the absence of telomere shortening, as in tumor cells in which telomerase is active, ageing is slowed and the transformed cells do not undergo senescence.[39]
Epidermal growth factor (EGF) activates telomerase by directly activating the telomerase reverse transcriptase (TERT).[40,41]
During senescence, low levels of EGF are observed. In addition to activating telomerase, EGF stimulates neurogenesis[42] and plays an important role in wound healing and the regeneration of tissues such as those of the skin, cornea and gastrointestinal tract.[43]
In summary, activation of the EGF receptor leads to an increase in maximum life expectancy, whereas decreases in the activity of the EGF pathway lead to an acceleration of ageing[44] and degeneration.[45]
EGF
Objective:
To modulate decreases in telomerase activity so as to prevent the critical shortening of telomeres, thereby favouring rejuvenation.
Dimethylsulfoxide (DMSO)
TERT[46] expression leads to telomerase activity and, thus, to an increase in the longevity of normal human cells, by decreasing their senescence.[47,48] As explained above, telomerase can reverse the degeneration of tissues provoked by ageing, in diverse organs, including the nervous system.[49]
However, it should not be forgotten that telomere dysfunction and telomerase activity are considered to be linked to the development of cancer.[50] On the one hand, gradual telomere shortening limits cell viability by favouring senescence,[51] but, on the other, the genomic instability triggered by telomere dysfunction is associated with a high risk of mutation, favouring tumour development.[52] Similarly, telomerase activity slows cell ageing, by favouring the regeneration of tissues, but it also contributes to the immortalization of transformed cells.[53,54]
Nerve Cells
Thus, for an anti-age intervention to be effective, it is essential to achieve a balance between these two age-related elements: ageing and tumour development. Various studies have highlighted the therapeutic value of a combination of TERT overexpression and tumour suppressor genes, notably p53 and p16, to stimulate anti-ageing activity whilst limiting cancer development.[55,56]
The p53 tumor suppressor gene, which has been described as “the guardian of the genome”,[57] encodes a transcription factor involved in control of the cell cycle, DNA repair, apoptosis and the response of the cell to stress. It can trigger cell growth arrest through direct activation of the cell cycle inhibitor p21 (inhibitor of the cyclin-dependent kinase, CDK).[58] The predominance of mutations inactivating the p53 tumour suppressor gene in most human cancers highlights the importance of this gene in the prevention of this disease.[59] However, by triggering cell growth arrest and apoptosis, p53 activates cell senescence and the ageing of the body.[60,61]
NF-kappa B is a direct antagonist of p53 that triggers cell survival, proliferation, migration and invasion.[62] During ageing, increases in NF-kappa B levels[63] are associated with cardiovascular diseases[64] and could result in oxidative stress[65] or a persistent inflammatory state, because inflammatory stimuli can induce the production of NF-kappa B.[66,67]
Dimethylsulfoxide (DMSO) has antioxidant,[68] cytoprotective[69] and anti-inflammatory properties.[70] In particular, DMSO has anti-tumour functions: it triggers the expression of the p53 tumor suppressor gene.[71,72] It also decreases levels of the c-myc proto-oncogene,[73] which is deregulated in most human tumors and contributes to malignant transformation by triggering uncontrolled cell proliferation and genomic instability.[74]
DMSO
Objective:
To activate tumour suppressor genes, such as p53, and to decrease the expression of proto-oncogenes, such as c-myc.
Specific Nucleic Acid SNA®-HLA I
NK cells can distinguish between normal cells and cells that have lost MHC class I molecule expression following viral infection or tumor transformation.[75] The p53 tumour suppressor gene activates cell senescence and prevents carcinogenesis. However, the NK cells must then eliminate the senescent tumours. p53 induces the secretion of chemokines by tumour cells; these molecules serve as signals for the recruitment of NK cells to the tumour environment.[76] However, the restoration of p53 does not increase the sensitivity of tumour cells to lysis by NK cells.[77] The cell lysis mediated by NK cells is more effective in the absence of MHC class I antigens. HLA-I antigens “complicate” the recognition of transformed cells for lysis by NK cells and interfere with various molecules involved in the lysis mediated by these cells.[78]
In conditions of stress, NK cell activity is down-regulated by glucocorticoids. Both lysis and fusion with cells sensitive to lysis are affected by glucocorticoids.[79]
SNA®-HLA I
Objective:
To inhibit the formation of HLA-I molecules, to favour the lytic response of NK cells.
Dehydroepiandrosterone (DHEA)
DHEA, which is secreted by the adrenal glands, is one of the most abundant steroids circulating in the human body. DHEA levels progressively decrease with age, suggesting a possible role in the ageing process. According to the neuroendocrine hypothesis of immuno-senescence, ageing and stress cause an imbalance in the cortisol/DHEA ratio, the principal determinant of the immunological changes observed in the elderly.[80] The decrease in DHEA secretion, together with an increase in cortisol secretion, results in greater exposure of the lymphoid cells to the deleterious effects of glucocorticoids.[1]
DHEA has immunostimulatory potential, and favours an increase in bone mineral density and cardiovascular and neurological protection.[81]
It also plays a major role in cell cycle control. DHEA prevents tumour progression by triggering cell senescence, inhibiting cell proliferation and increasing cell death by apoptosis. The effects of DHEA on cells are associated with an increase in the expression of the cell cycle inhibitor genes p16 and p21.[82] The p21 protein (which inhibits CDK, also known as p21WAF1 / Cip1) favours cell cycle arrest in response to numerous stimuli.[83] The p16 gene is also a major tumour suppressor gene. The high frequency of p16 deletions in primary tumour cell lines suggests an important role for p16 in carcinogenesis; indeed, p16 loss is frequently reported to be an early and often critical event in tumour progression.[84]
DHEA
Objective:
To restore the balance in glucocorticoid levels (altered cortisol/DHEA ratio) and to stimulate the expression of cell cycle inhibitor genes (such as the p21 and p16 genes), to prevent tumorigenic cell proliferation.
The MISEN Formula: Action at Several Different Levels
- Hormonal balance and immune defence;
- Cell regeneration and rejuvenation;
- Antitumour capacity;
- DHEA - Restoring the balance of glucocorticoid levels;
- SNA-HLAII - Preventing immune depletion;
- IL-2 / RNA - Modulating the decrease in CD28 expression by T lymphocytes;
- SNAH-MISEN / EGF - Modulating telomerase activity;
- CMSO - Activating tumour suppressor genes (p53) / decreasing the expression of proto-oncogenes (c-myc);
- SNA-HLAI - Activating NK cells;
- DHEA - Stimulating cell cycle inhibitors (p21, p16).
III. Conclusion
The MISEN formula is designed to act on several different physio-pathological mechanisms linked to chronic stress and ageing, with the following objectives:
- To prevent the immune depletion occurring during senescence (whether natural or induced by chronic stress) and to increase immune defence capacity;
- To counteract the pro-inflammatory effects of diverse factors;
- To favour cell regeneration and rejuvenation, by preventing decreases in the activity of telomerase and associated factors;
- To increase, in parallel, the antitumour and antiproliferative capacities of the body.
In summary, the MISEN formula aims to confer a better response of the immune system and a balance between the processes of senescence and cell proliferation.
References and Bibliography
1. Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. Ann N Y Acad Sci. 1153: 139-52; 2009.
2. Butcher SK, Lord JM. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 3(4): 151-60; 2004.
3. Correia-Melo C, Hewitt G, Passos JF.Telomeres, oxidative stress and inflammatory factors: partners in cellular senescence? Longev Healthspan. 3(1): 1; 2014.
4. Freund A et al. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 16(5): 238-46; 2010.
5. Muntasell A et al. HLA-DR4 molecules in neuroendocrine epithelial cells associate to a heterogeneous repertoire of cytoplasmic and surface self peptides. J Immunol. 169(9): 5052-60; 2002.
6. Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 12: 259-93; 1994.
7. Viret C, Janeway Jr CA. MHC and T cell development. Rev Immunogenet. 1: 91-104; 1999.
8. Ting JP, Trowsdale J. Genetic control of MHC class II expression. Cell. 109: S21-33; 2002.
9. Jin HT et al. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 44(4): 217-31; 2011.
10. Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol. 30(6): 798-805; 2010.
11. Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 30(7): 306-12; 2009.
12. Martínez-Llordella M et al. CD28-inducible transcription factor DEC1 is required for efficient autoreactive CD4+ T cell response. J Exp Med. 210(8): 1603-19; 2013.
13. Appleman LJ et al. CD28 costimulation mediates T cell expansion via IL-2-independent and IL-2-dependent regulation of cell cycle progression. J Immunol. 164(1): 144-51; 2000.
14. Thompson CB et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA. 86(4): 1333–1337; 1989.
15. Nasir Salam et al. T cell ageing: Effects of age on development, survival & function. Indian J Med Res. 138(5): 595–608; Nov 2013.
16. Mariani E et al. Different rates of telomere shortening and telomerase activity reduction in CD8 T and CD16 NK lymphocytes with ageing. Exp Gerontol. 38(6): 653-9; 2003.
17. Hug N, Lingner J. Telomere length homeostasis. Chromosoma. 115(6): 413-25; 2006.
18. Kaszubowska L. Telomere shortening and ageing of the immune system. J Physiol Pharmacol. 59 Suppl 9: 169-86; 2008.
19. Xu W et al. Ref-1 protein enhances the IL-2-stimulated telomerase activity. J Cell Biochem. 88(6): 1120-8; 2003.
20. Kawauchi K, Ihjima K, Yamada O. IL-2 increases human telomerase reverse transcriptase activity transcriptionally and posttranslationally through phosphatidylinositol 3’-kinase/Akt, heat shock protein 90, and mammalian target of rapamycin in transformed NK cells. J Immunol. 174(9): 5261-9; 2005.
21. Bazhan SI, Belova OE. Molecular genetic aspects of interferon induction and antiviral action. Vestn Ross Akad Med Nauk. (3): 18-24; 1998.
22. Srivastava S, Koch LK, Campbell DJ. IFNαR Signaling in Effector but Not Regulatory T Cells Is Required for Immune Dysregulation during Type I IFN-Dependent Inflammatory Disease. J Immunol. 193(6): 2733-42; 2014.
23. Lanna A et al. IFN-α inhibits telomerase in human CD8⁺ T cells by both hTERT downregulation and induction of p38 MAPK signaling. J Immunol. 191(7): 3744-52; 2013.
24. Reed JR et al. Telomere erosion in memory T cells induced by telomerase inhibition at the site of antigenic challenge in vivo. J Exp Med. 199(10): 1433-43; 2004.
25. Lindkvist A et al. Interferon-induced sensitization to apoptosis is associated with repressed transcriptional activity of the hTERT promoter in multiple myeloma. Biochem Biophys Res Commun. 341:1141–1148; 2006.
26. Xu D et al. Interferon alpha down-regulates telomerase reverse transcriptase and telomerase activity in human malignant and nonmalignant hematopoietic cells. Blood. 96: 4313–4318; 2000.
27. Hornung V et al. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 173(10): 5935-43; 2004.
28. Lan T et al. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci U S A. 104(34): 13750-5; 2007.
29. Dai J et al. Regulation of IFN regulatory factor-7 and IFN-alpha production by enveloped virus and lipopolysaccharide in human plasmacytoid dendritic cells. J Immunol. 173(3): 1535-48; 2004.
30. Seya T, Shingai M, Matsumoto M. Toll-like receptors that sense viral infection. Uirusu 54(1): 1-8; 2004.
31. Heil F et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 303(5663):1526-9; 2004.
32. Diebold SS et al. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 303(5663): 1529-31; 2004.
33. Lund JM et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 101(15): 5598-603; 2004.
34. Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 120(4): 449-60; 2005.
35. Antebi A. Genetics of aging in Caenorhabditis elegans. PLoS Genet. 3(9): 1565-71; 2007.
36. Smith-Vikos T, Slack FJ. MicroRNAs and their roles in aging. J Cell Sci. 125(Pt 1): 7-17; 2012.
37. Campisi J. Cancer, aging and cellular senescence. In Vivo 14(1): 183-8; 2000.
38. Buchkovich KJ. Telomeres, telomerase, and the cell cycle. Prog Cell Cycle Res. 2:187-95; 1996.
39. Mikhelson VM, Gamaley IA. Telomere shortening is a sole mechanism of aging in mammals. Curr Aging Sci. 5(3): 203-8; 2012.
40. Maida Y et al. Direct activation of telomerase by EGF through Ets-mediated transactivation of TERT via MAP kinase signaling pathway. Oncogene 21(26): 4071-9; 2002.
41. Salehinejad P et al. Effect of EGF and FGF on the expansion properties of human umbilical cord mesenchymal cells. In Vitro Cell Dev Biol Anim. 49(7): 515-235; 2013.
42. Enwere E et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 24(38): 8354-65; 2004.
43. Schultz G, Rotatori DS, Clark W. EGF and TGF-alpha in wound healing and repair. J Cell Biochem. 45(4): 346-52; 1991.
44. Yu S, Driscoll M. EGF signaling comes of age: promotion of healthy aging in C. elegans. Exp Gerontol. 46(2-3):129-34; 2011.
45. Siddiqui S et al. Central role of the EGF receptor in neurometabolic aging. Int J Endocrinol. 2012: 739428; 2012.
46. Zhou J et al. Telomerase reverse transcriptase in the regulation of gene expression. BMB Rep. 47(1): 8-14; 2014.
47. Bodnar AG et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 279: 349–352; 1998.
48. Vaziri H, Benchimol S. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr Biol. 8: 279–282; 1998.
49. Jaskelioff M et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 469: 102–106; 2011.
50. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144: 646–674; 2011.
51. Flores I, Benetti R, Blasco MA. Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol. 18: 254–260; 2006.
52. Feldser DM, Hackett JA, Greider CW. Telomere dysfunction and the initiation of genome instability. Nat Rev Cancer. 3: 623–627; 2003.
53. Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 33: 787–791; 1997.
54. Belair CD et al. Telomerase activity: a biomarker of cell proliferation, not malignant transformation. Proc Natl Acad Sci U S A. 94: 13677–13682; 1997.
55. Hartwig FP et al. Up-regulating telomerase and tumor suppressors: focusing on anti-aging interventions at the population level. Aging Dis. 5(1): 17-26; 2013.
56. Tomás-Loba A et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell 135(4): 609-22; 2008.
57. Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 60(24): 6788-93; 2000.
58. Eckner R. p53-dependent growth arrest and induction of p21: a critical role for PCAF-mediated histone acetylation. Cell Cycle. 11(14): 2591-2; 2012.
59. Mendrysa SM, Perry ME. Tumor suppression by p53 without accelerated aging: just enough of a good thing? Cell Cycle 5(7): 714-7; 2006.
60. Rufini A et al. Senescence and aging: the critical roles of p53. Oncogene 2013; 32(43): 5129-43.
61. Hasty P, Christy BA. p53 as an intervention target for cancer and aging. Pathobiol Aging Age Relat Dis. 3; 2013.
62. Pal S et al. Chronic inflammation and cancer: potential chemoprevention through nuclear factor kappa B and p53 mutual antagonism. J Inflamm (Lond). 11:23; 2014.
63. Hajra L et al. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 97: 9052–9057; 2000.
64. Bourcier T, Sukhova G, Libby P. The nuclear factor kappa-B signaling pathway participates in dysregulation of vascular smooth muscle cells in vitro and in human atherosclerosis. J Biol Chem. 272: 15817–15824; 1997.
65. Donato AJ et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 100: 1659–1666; 2007.
66. Liu H et al. Redox-dependent transcriptional regulation. Circ Res. 97: 967–974; 2005.
67. Ungvari Z, Csiszar A, Kaley G. Vascular inflammation in aging. Herz 29: 733–740; 2004.
68. Sanmartín-Suárez C et al. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J Pharmacol Toxicol Methods. 63(2): 209-15; 2011.
69. Man W et al. Dimethyl sulfoxide attenuates hydrogen peroxide-induced injury in cardiomyocytes via heme oxygenase-1. J Cell Biochem. 115(6): 1159-65; 2014.
70. Jacob SW, Herschler R. Pharmacology of DMSO. Cryobiology. 23(1): 14-27; 1986.
71. Koiri RK, Trigun SK. Dimethyl sulfoxide activates tumor necrosis factorα-p53 mediated apoptosis and down regulates D-fructose-6-phosphate-2-kinase and lactate dehydrogenase-5 in Dalton’s lymphoma in vivo. Leuk Res. 35(7): 950-6; 2011.
72. Menendez D et al. Diverse stresses dramatically alter genome-wide p53 binding and transactivation landscape in human cancer cells. Nucleic Acids Res. 41(15): 7286-301; 2013.
73. Darling D et al. DMSO induced modulation of c-myc steady-state RNA levels in a variety of different cell lines. Oncogene. 4(2): 175-9; 1989.
74. Wahlström T, Arsenian Henriksson M. Impact of MYC in regulation of tumor cell metabolism. Biochim Biophys Acta. pii: S1874-9399(14)00192-8; 2014.
75. Le Maux Chansac B et al. NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol. 175(9): 5790-8; 2005.
76. Iannello A et al. p53-dependent chemokine production by senescent tumor cells supports NKG2D-dependent tumor elimination by natural killer cells. J Exp Med. 2013; 210(10):2057-69.
77. Xue W et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 445(7128): 656-60; 2007.
78. Peña J, Solana R. Histocompatibility antigens and natural killer susceptibility. Immunol Res. 11(2): 133-40; 1992.
79. Matera L et al. Effect of cortisol on the native and in vitro induced non-MHC restricted cytotoxicity of large granular lymphocytes. J Clin Lab Immunol. 27(2): 77-81; 1988.
80. Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 8(1): 69-83; 2005.
81. Barrou Z, Charru P, Lidy C. Actions of dehydroepiandrosterone: possible links with aging. Presse Med. 25(38): 1885-9; 1996.
82. Shilkaitis A et al. Dehydroepiandrosterone inhibits the progression phase of mammary carcinogenesis by inducing cellular senescence via a p16-dependent but p53-independent mechanism. Breast Cancer Res. 7(6): R1132-40. 2005.
83. Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 9(6): 400-14. 2009.
84. Rocco JW, Sidransky D. p16(MTS-1/CDKN2/INK4a) in cancer progression. Exp Cell Res. 264(1): 42-55. 2001.
Comments:
-
Dr. Peter H Kay said..
Thank you Dr. Reig. I read your article with interest. I would be grateful if you could help me understand more about the workings of remedies that include SNAs (specific nucleic acids). I would also like to raise some potential safety issues associated with the use of these specific SNAs.
Firstly, I cannot understand how oral administration of small, presumably single stranded DNA or RNA molecules which are diluted to such an extent that they do not actually contain any nucleic acid molecules, can reduce the number of MHC class 1 and class 2 molecules on the surface of cells. Could you please explain how SNAs do this? Further, there are a number of quite different MHC class 1 as well as class 2 molecules such as HLA A, B, C, D (DR, DQ and DP), E and others. These HLA molecules have quite different functions. Which MHC class 1 and class 2 HLA molecule/s is/are targeted by the SNAs in the remedy you describe? Safety considerations.
Many of the different types of HLA molecules such as those derived from the HLA B and DR loci, for example, display remarkable genetic polymorphism. It is well established that some genetic variants of different HLA cell surface molecules are associated with disease whilst the presence of others is associated with protection against disease. Bearing in mind these differences, it would seem helpful to determine an individual's HLA B and/or DR/DQ genetic types at least before administration of a remedy that you describe. Reduction of MHC class 1 or 2 molecules that are associated with disease protection would be contra-indicated.
-
Dr Lourdes Reig said..
Dear Dr. Kay, we thank your for your interest and we hope to answer your queries regarding MI.
With reference to your first question and basing our approach systematically on experimentation and scientific evidence collected data, we confirm that the potential security risks associated with Ultra High Dilution, infinitesimal dilutions or homeopathic nature dilutions, are often minimal or non-existent. i ii
As you quote and as we confirm, our therapy is based on the combination of various individual molecules that are diluted to the point that the analysis of highly diluted substances, in this case, the SNA, in reality, appears not to contain any “measurable” molecules.
It is well established that cells express cellular genes pathways, and that they are likely to be modified through immune responses and signalling molecules such as cytokines, chemokines, neurotrophic factors, etc. Furthermore, these signalling molecules have a very defined effect depending on the environment of cytokines in which they are. We proceed from this approach/concept to justify our medicines, and the observations we make are established on the results obtained through therapeutic effects. Our explanation is that the effect of these highly diluted substances could be the result of a regulatory action at a molecular level, through epigenetic modulations and the expression of various genes involved in different pathologies.
We also rely on the fact that nowadays, there is sufficient scientific evidence to suggest molecular actions derived from highly diluted molecules, and that also, through well-designed experiments, scientific research corroborates these regulatory functions at the level of relevant genes expression, presumably by epigenetic modification. iii iv v vi vii viii ix x xi xii
Regarding the specific issue of oral administration of DNA or single-stranded RNA molecules, the matter is less complex, since we agreed that actually these oligonucleotides were physiological molecules which the body is accustomed to, and which exert actions in nanomolar minimal amounts. xiii There are studies about the evidence of the epigenetic effects of nucleic acids through the modulation of Toll-Like receptor, cytokine secretion such as IFN-alpha and other actions. xiv xv In both cases, the ssDNA and ssRNA of our formulas are prepared in moderate dilution in order to obtain modulatory effects.
In the case of HLA-I molecules, the evidence shows that in many diseases we deal with, viruses, bacteria etc., manipulating HLA class 1 ensures their latency. The presence of major histocompatibility antigens (MHC), especially class I antigens, inhibits mediated lysis by NK cells. xvi Similarly, defects in numbers and / or function of NKT cells are observed in a variety of animals, genetically inclined in the development of autoimmune diseases. xvii In very specific and well-defined pathologies, our medicines only contain structures such as anti-HLA SNA in inhibitory high dilution, in order to promote the development of these structures and enhance with them the cell lysis by NK cells. It should be highlighted that these highly diluted SNA, are only found in specific sequences, in minimum dose, for example once every ten days, with a well-studied cytokine environment to generate a specific action and especially in formulas such as autoimmunity, cancer... etc. where the action would be fully justified.
In the case of HLA-II (DR, DQ or DP), it is clear that they have very different functions compare to the HLA-I functions, and that these structures are essential for immune response when presenting in antigen-presenting cells, such as dendritic cells, B cells and macrophages, therefore its expression is not justified systematically. Our medicines do not contain these structures expressed in professional APCs.
When under IFN gamma induction, xviii molecules such as HLA-II express themselves constitutionally in non-professional cells such as fibroblasts, astrocytes, endothelial cells, epithelial cells, pancreatic cells, thyroid cells, T cells, etc. not to express the full range of costimulatory molecules, xix there is a direct relationship with a wide range of diseases. Structures found in our medicines are only expressed in non-professional APCs such as SNA HLA-II in inhibitory high dilution in order to reduce expression of these specific molecules in definite cells, since genetic sequences in these molecules expressed in different non-professional cells are currently known.
This is for example the case of aberrant expression of HLA-DR in thyroid cells, which may initiate autoimmunity, by way of facultative antigen presentation as APCs. xx xxi Aberrant expression of HLA-DR in salivary glands in the development of Sjögren's syndrome, xxii the expression of HLA-DQ in pancreatic cells and predisposition to diabetes, xxiii increased endothelial expression of HLA-II (DQ, DR, DP) evidenced in autoimmune diseases, such as myocarditis with dilated cardiomyopathy, rheumatoid arthritis and systemic lupus erythematosus. xxiv Similarly, transplant rejection strictly depends on MHCII’s expression in non-professional APCs such as endothelial and epithelial cells. xxv
In the case in question, "Stress and Ageing", without indicating exactly which molecule and HLA-I and II types are included in the formula specified in the article, we can say that the choice of one or the other has been consecutive to comprehensive studies on different pathologies. In the case of HLA-I, they are aimed at stimulating immunity directed by NK cells, and HLA-II indicate that there is evidence suggesting that infections by chronic pathogens presented by non-professional APCs, may eventually cause immunosuppression events favouring the state of immune senescence and anergy of T cells. xxvi It is obvious that we are not intending to reduce HLA type 1 or 2 molecules, associated with disease protection.
Finally, I apologize for the length of my answer, as the explanation can only be objective under a scientific prism. We think that medicine is in need of a multidisciplinary approach, as it could provide many options against different pathologies, and we are convinced that scientific advances will really prove the value of these substances and their mechanisms in a very near future.
I. Endrizzi C1, Rossi E, Crudeli L, Garibaldi D. Harm in homeopathy: aggravations, adverse drug events or medication errors? Homeopathy. 2005 Oct;94(4):233-40.
II. Dantas F, Rampes H. Do homeopathic medicines provoke adverse effects? A systematic review. Br Homeopath J. 2000;89(Suppl. 1):S35–38
III. Marzotto Saha SK1, Roy S2, Khuda-Bukhsh AR1. Ultra-highly diluted plant extracts of Hydrastis canadensis and Marsdenia condurango induce epigenetic modifications and alter gene expression profiles in HeLa cells in vitro. J Integr Med. 2015 Nov;13(6):400-11. doi: 10.1016/S2095-4964(15)60201-1.
IV. Dei A1, Bernardini S2. Hormetic effects of extremely diluted solutions on gene expression. Homeopathy. 2015 Apr;104(2):116-22. doi: 10.1016/j.homp.2015.02.008. Epub 2015 Mar 21.
V. M, Olioso D, Brizzi M, Tononi P, Cristofoletti M, Bellavite P1. Extreme sensitivity of gene expression in human SH-SY5Y neurocytes to ultra-low doses of Gelsemium sempervirens. BMC Complement Altern Med. 2014 Mar 19;14:104. doi: 10.1186/1472-6882-14-104.
VI. Marzotto M1, Olioso D1, Bellavite P1. Gene expression and highly diluted molecules. Front Pharmacol. 2014 Nov 12;5:237. doi: 10.3389/fphar.2014.00237. eCollection 2014.
VII. Bigagli E1, Luceri C2, Bernardini S3, Dei A4, Filippini A2, Dolara P2.
Exploring the effects of homeopathic Apis mellifica preparations on human gene expression profiles. Homeopathy. 2014 Apr;103(2):127-32. doi: 10.1016/j.homp.2014.01.003.
VIII. Samadder A1, Das S, Das J, Paul A, Boujedaini N, Khuda-Bukhsh AR. The potentized homeopathic drug, Lycopodium clavatum (5C and 15C) has anti-cancer effect on hela cells in vitro. J Acupunct Meridian Stud. 2013 Aug;6(4):180-7. doi: 10.1016/j.jams.2013.04.004. Epub 2013 Apr 28.
IX. Paul, S., Mandal, S.K., Bhattacharyya, S.S., Boujedaini, N., and Khuda-Bukhsh, A.R. In vitro and in vivostudies demonstrate anticancer property of root extract of Polygala senega. J Acupunct Meridian Stud. 2010; 3: 188–196
X. Biswas, S.J., Bhattacharjee, N., and Khuda-Bukhsh, A.R. Efficacy of a plant extract (Chelidonium majus L.) in combating induced hepatocarcinogenesis in mice. Food Chem Toxicol. 2008; 46: 1474–1487
XI. Pathak, S., Das, J.K., Biswas, S.J., and Khuda-Bukhsh, A.R. Protective potentials of a potentized homeopathic drug, Lycopodium-30, in ameliorating azo dye induced hepatocarcinogenesis in mice.Mol Cell Biochem. 2006; 285: 121–131
XII. Wang, X., Martindale, J.L., and Holbrook, N.J. Requirement for ERK activation in cisplatin-induced apoptosis. J Biol Chem. 2000; 275: 39435–39443.
XIII. Unterholzner L1. The interferon response to intracellular DNA: why so many receptors?
Immunobiology. 2013 Nov;218(11):1312-21. doi: 10.1016/j.imbio.2013.07.007. Epub 2013 Jul 29.
XIV. Diebold SS1. Recognition of viral single-stranded RNA by Toll-like receptors. Adv Drug Deliv Rev. 2008 Apr 29;60(7):813-23. doi: 10.1016/j.addr.2007.11.004. Epub 2007 Dec 27.
XV. Han X1, Li X, Yue SC, Anandaiah A, Hashem F, Reinach PS, Koziel H, Tachado SD. Epigenetic regulation of tumor necrosis factor ? (TNF?) release in human macrophages by HIV-1 single-stranded RNA (ssRNA) is dependent on TLR8 signaling. J Biol Chem. 2012 Apr 20;287(17):13778-86. doi: 10.1074/jbc.M112.342683. Epub 2012 Mar 5.
XVI. Horton NC1, Mathew SO, Mathew PA. Novel interaction between proliferating cell nuclear antigen and HLA I on the surface of tumor cells inhibits NK cell function through NKp44. PLoS One. 2013;8(3):e59552. doi: 10.1371/journal.pone.0059552. Epub 2013 Mar 19.
XVII. Mieza, M.A., T. Itoh, J.Q. Cui, Y. Makino, T. Kawano, K. Tsuchida, T. Koike, T. Shirai, H. Yagita, A. Matsuzawa, et al. 1996. Selective reduction of V?14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 156:4035–4040.
XVIII. Sprent J. Antigen-presenting cells. Professionals and amateurs. Curr Biol. 1995 Oct 1;5(10):1095-7.
XIX. Pisapia L1, Pozzo GD1, Barba P1, Citro A2, Harris PE2, Maffei A3. Contrasting effects of IFN? on MHC class II expression in professional vs. nonprofessional APCs: Role of CIITA type IV promoter. Results Immunol. 2012 Sep 27;2:174-83. doi: 10.1016/j.rinim.2012.09.001. eCollection 2012.
XX. Hanafusa T, Pujol Borrell R, Chiovato L, Russell RC, Doniach D, Bottazzo GF. Aberrant expression of HLA-DR antigen on thyrocytes in Graves’ disease: relevance for autoimmunity. Lancet. 1983;2:1111–1115
XXI. Bottazzo GF, Pujol Borrell R, Hanafusa T, Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;2:1115–1119.
XXII. Tsunawaki S1, Nakamura S, Ohyama Y, Sasaki M, Ikebe-Hiroki A, Hiraki A, Kadena T, Kawamura E, Kumamaru W, Shinohara M, Shirasuna K. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjögren's syndrome. J Rheumatol. 2002 Sep;29(9):1884-96.
XXIII. Harris P.E., Malanga D., Liu Z., Hardy M.A., Souza F., Del Pozzo G., Winchester R.J., Maffei A. Effect of interferon alpha on MHC class II gene expression in ex vivo human islet tissue. Biochimica et Biophysica Acta. 2006;1762:627–635
XXIV. Turesson C1. Curr Pharm Des. 2004;10(2):129-43. Endothelial expression of MHC class II molecules in autoimmune disease.
XXV. Game D.S., Lechler R.I. Pathways of allorecognition: implications for transplantation tolerance.Transplant Immunology. 2002;10:101–108.
XXVI. Jin HT1, Jeong YH, Park HJ, Ha SJ. Mechanism of T cell exhaustion in a chronic environment. BMB Rep. 2011 Apr;44(4):217-31.DOI 10.5483/BMBRep.2011.44.4.217.
-
Dr. Peter H Kay said..
Thank you Dr. Reig. Unfortunately, your reply does not respond effectively to my queries. I will communicate directly with Labo'Life to discuss these matters further.