Positive Health Online
Your Country

Management and Treatment of ME - Part I
listed in cfs me long covid, originally published in issue 57 - October 2000
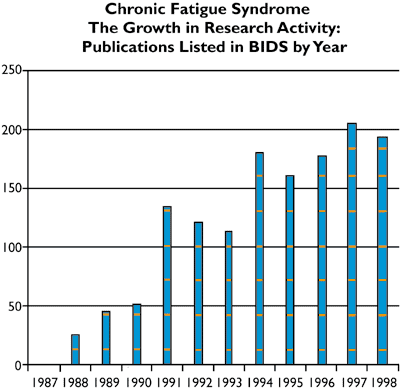
The number of research publications on CFS/ME has grown very dramatically in recent years. The number of publications listed by year in BIDS (the Bath Information and Data Service) has increased from none at all prior to 1987 to around 200 per year – www.doh.gov.uk/cfs-discuss.htm
ME is a condition which is increasingly recognised in the UK. It is thought that there are 150,000 – 200,000 people with the condition in the British population, though epidemiological estimates vary about eight-fold. A recent community-based survey in Chicago suggested that it affected about 0.42% of the population.[1] This proportion applied to the UK population would suggest in excess of 200,000 people with ME in Britain, in line with the upper end of the estimated range, but considerable care has to exercised in applying figures obtained in a different population, because there is likely to be considerable natural variation between populations.
People with ME have suffered over many years as a result of a lack of knowledge of the disease among many health care professionals, accompanied in many cases by lack of sympathy, understanding or belief. There is now a window of opportunity for change, with the recognition by the government of the importance of the illness, and the appointment by the Chief Medical Officer of a Working Group to draw up guidance for clinicians on what sort of clinical care to provide for ME sufferers. The Working Group started work at the beginning of 1999, and its report should be available in mid-2001.
An initial scoping document for the Working Group underlined the vast upsurge in research activity that has taken place in recent years. This is reflected in a large increase, year on year, in the number of research papers published. (See Fig. 1)
Underlying Disease Processes and Therapy
While the clinical features of chronic fatigue syndrome are fairly unspecific, numerous pathophysiological changes have been demonstrated which, increasingly, enable the condition to be distinguished from other disorders with which it has in the past at times been confused. Such changes have been demonstrated affecting, for example, the autonomic nervous system.[2] Another significant change is in the hypothalamic-pituitary axis, the mechanism by which the brain controls the functioning of the pituitary gland and thence the endocrine system. Recent studies involving a method known as the buspirone challenge test suggest increased activity, or upregulation, of 5-hydroxytryptamine receptors in the hypothalamus of patients with ME but not in people with primary depression, indicating a clear distinction between these two conditions.[3]
Impaired activation of the hypothalamic-pituitary-adrenal (HPA) axis is an essential neuroendocrine feature of CFS. Patients with CFS have a reduction of HPA axis activity due, in part, to impaired central nervous system drive.[4] These observations provide an important clue to the development of more effective treatment to this disabling condition.
An atypical immune response to persistent viruses or viral antigens has long been thought likely to be involved in the development of ME,[5] but the mechanisms involved are clearly complex, and some recent research has failed to confirm either the presence of persistent viral infection,[6] or of either immune activation[7] or significant impairment of cell-mediated immunity.[8] However, one small-scale study has indicated a good response among a subset of ME to ganciclovir, a specific antiviral treatment for cytomegalovirus.[9]
Immune Dysfunction
The use of immunoglobulin in ME to overcome problems attributable to immune dysfunction has been the focus of a number of studies. An early study in Germany suggested that this had the most potential of a number of treatments examined.[10] However, studies in Australia of the use of immunoglobulins for the treatment of ME have yielded conflicting results. One double-blind randomised controlled trial in adolescents showed significant improvement at six months,[11] but longer term follow up and three and five years showed substantial numbers still disabled.[12] Another study found no evidence of benefit in adult patients, though, and a high (70-80%) incidence of side effects.[13] This confirms earlier work reporting a lack of response to either immunological or psychological therapy when used in combination.[14] Similarly, an American study[15] failed to confirm improvements noted in an Australian report.[16] Another possible approach which has been proposed for enhancing the immune system is through the use of Chinese medicine,[17] though this has yet to be the subject of published clinical trials. The possibility that staphylococcus toxoid vaccine may be beneficial through its general stimulating effect on the immune system has led to one clinical trial, with encouraging results.[18]
Attempts have been made to generate integrated hypotheses for the causation of CFS, often involving cytokines, which are growth factors secreted mainly by white blood cells or leucocytes. They are responsible for stimulating the body's defences against disease, particularly its immune responses. One such mechanism involves viruses or bacteria inducing production of various cytokines known as interleukins and interferons. These in turn cause increased production of nitric oxide by enhancing production of the enzyme nitric oxide synthase. The model then involves nitric oxide reacting with the superoxide radical to generate the potent oxidant peroxynitrite. This may affect the hypothalamic-pituitary axis.[19]
Diet and Nutrition
Such a model, if correct, would create the prospect of a potential role for dietary antioxidants in the prevention and treatment of ME. This is just one example of ways in which increasing knowledge of the underlying mechanisms operating in ME have led to trials of increasing numbers of interventions, with varying results. Thus, various marginal nutritional deficiencies have been suggested as related to the causation of CFS, including lack of various B vitamins, vitamin C, magnesium, sodium, zinc, L-tryptophan, L-carnitine, coenzyme Q10, and essential fatty acids.[20] As regards these latter, the research evidence is conflicting, with one study suggesting improvement among 85% of patients receiving essential fatty acids, compared with just 17% receiving a placebo,[21] but a subsequent report failed to confirm this.[22]
Magnesium deficiency has been cited as another possible causal factor, and reduced red blood cell concentrations have been found in patients with CFS. A small randomized placebo-controlled trial of magnesium supplements did appear to indicate symptomatic improvement in subjects treated with magnesium,[23] but an investigation of red blood cell magnesium concentrations in ME patients and normal controls found no significant difference between the two groups.[24] The parallel has been drawn between CFS and the latent tetany syndrome, where magnesium loss occurs as a result of increased stress-related adrenaline and noradrenaline release.[25]
An empirical observation, that carnitine levels are reduced in CFS, has led to another therapeutic approach. A small clinical trial[26] compared oral L-carnitine with amantadine, which has been shown to be effective in treating the fatigue of multiple sclerosis, though not in ME.[27] While amantadine was poorly tolerated, L-carnitine administration was associated with significant clinical improvement. However, other studies are required to confirm this observation. Another suggestion, related to the presumed causation of CFS, is that CFS may be related to viral damage to muscle cells causing calcium ions to enter them in greater numbers than usual, in which case calcium antagonists may be useful in treating CFS.[28]
L-Arginine, an essential amino acids, is another dietary factor which may benefit ME, perhaps by the same mechanism reported by Pall9, since its metabolism involves nitric oxide production. It has an effect on the immune system by activating transformed lymphocytes called Natural Killer (NK) cells, and this effect is mediated by nitric oxide.[29] This may account for marked improvements seen in an uncontrolled trial of mixtures of dietary amino-acids in patients with ME.[30]
The question of diet in general has been a major concern for many people with ME, but apart from the specific examples cited above, the research findings are equivocal. A placebo-controlled, cross-over study of vitamin and mineral supplementation, for example, suggested some improvement in the treatment group, but statistical analysis indicated that the effect of treatment was not significant.[31] A study of intramuscular bovine liver extract containing folic acid and cyanocobalamin showed no improvement compared with placebo.[32] A number of dietary theories have been put forward over the years, often based on anecdotal evidence, but a review of some of these asserted that there was little evidence to support them, for example the use of yeast-avoidance and sugar-free diets, to combat Candida albicans infection. It concluded that a varied diet with all the major food groups represented was the most sensible approach to adopt in ME.[33]
Persistent Viral Infection
The likelihood that persistent virus infection is important in ME has stimulated a number of studies of anti-viral transfer factor (TF) in its treatment. A case report of two patients treated with TF indicated that one had shown improvement and the second had not.[34] This may indicate that the disease is heterogeneous, and that different causal factors are to be found in different cases.[35] Thus a placebo-controlled pilot study of TF in 20 patients showed improvements in 12, but not in all, of the patients.[36] Another large study showed a decreasing success rate with TF with increasing age of the patient, again pointing to the disease's heterogeneity.[37]
Another approach to specific antiviral therapy is through the use of interferon. A clinical trial involving thirty patients treated with alpha 2a interferon or placebo in a double-blind crossover study demonstrated that the quality of life score improved markedly only in seven patients with NK cell dysfunction, again underlining the heterogeneity of the condition, and suggesting that there is a specific subgroup of patients with CFS who manifest this feature, which is not found in other patients.[38]
Another approach relying on augmentation of NK cell activity is through the use of ampligen, a mismatched double-stranded RNA molecule, which works by correcting reductions in latent 2-5A synthetase and elevations in RNase L, changes which are associated with reactivation of viruses such as HHV-6 (human herpes virus) or Epstein-Barr virus (associated with glandular fever).[39] A small scale uncontrolled study of 15 patients treated with ampligen showed decreased virus activation associated with marked clinical improvements.[40]
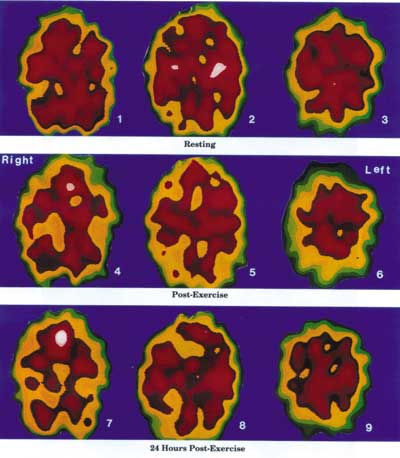
The Negative Effects of Exercise on an ME/CFS Dysfunctional Brain
Resting State Images 1, 2 and 3, represent the abnormal resting state of an ME/CFS brain. There is a perfusion defect in the left interior frontal lobe as well as the right and left posterior parietal lobes.
Immediate Post-Exercise State Images 4, 5 and 6 represent the immediate post-exercise function of the brain of the same patient. There is a significant decrease in perfusion of the right and left frontal lobes and the right and left posterior parietal lobes. An occipital perfusion defect is starting to appear. The functional resting defects noted in images 1, 2 and 3 have become aggravated.
24 Hours Post-Exercise State Images 7, 8 and 9 illustrate the severely decreased brain perfusion of the same patient 24 house after the brain has been stressed by physical exercise. This 24 hour delayed effect may explain much of the ME/CFS dysfunction that occurs the day after exercise or other stress factors.
The Xeon SPECT scans of a 37 year old female ME/CFS patient and the concept were provided by Dr Jay Goldstein of Anaheim, California. The technical expertise is that of Dr Ismael Mena, UCLA Harbor, California.}
Anti-Inflammatory Treatment
The use of steroids such as hydrocortisone in ME has been prompted by two considerations, firstly the observation that plasma cortisol concentrations tend to be low in ME, in contrast to what is found in depression, and secondly the fact that cytokines have been implicated in the causation of ME and may stimulate inflammation, suggesting that anti-inflammatory drugs such as steroids may have a role in treatment. Two randomized controlled trials have indicated some improvement among patients treated with low dose hydrocortisone,[41] but in one of these the point was made that the associated adrenal gland suppression made the treatment impractical,[42] while another study, of the use of oestrogens, has suggested some benefit to women with ME.[43] A placebo-controlled, double-blind, random-allocation crossover trial of fludrocortisone did not show any benefit, though the point has to be made that this was conducted among attenders at an outpatient unit, and therefore excluded the most severe cases, underlining once again the heterogeneity of the disease, and the need to test potential new treatments in the most appropriate subgroups and not to generalize from the results to the whole population with ME.[44] Treatment with another steroid, dehydroepiandrosterone (DHEA), has been shown to reduce the symptoms of ME in those patients with low DHEA production,[45] and a pilot study in Japan did show some benefit from DHEA when combined with vitamin C.[46] However, this does not apply to all patients, underlying the importance of identifying specific subgroups of ME in order to interpret the outcomes of treatment trials.
The antihistamine terfenadine has also been the subject of a randomized controlled trial, as an agent of potential use in the treatment of ME because of its anti-inflammatory properties, but was not found to be of benefit.[47] A more direct approach would involve treatment with cytokine blockers,[48] but this has yet to be carried out.
The Central Nervous System
The central nervous system manifestations of ME create other opportunities for potentially effective therapeutic interventions. Studies of blood perfusion of the brain have revealed characteristic blood flow reductions in many regions, and in particular in that part of the brain stem known as the reticular activating system.[49] Similar lesions are found in the post-polio syndrome, with which ME is comparable in many ways. This means that ultimately it should be possible to target therapy to those regions of the brain which are particularly susceptible to damage in ME, opening the door to much more specific treatment than is currently available. Of more immediate value are other studies taking as their starting point the likely changes in brain chemistry occurring in ME. Thus one study was based on the suggestion that, because ME mimics the effects of the drug reserpine, a drug designed to nullify its effects could be useful in ME. The drug in question was the monoamine oxidase inhibitor phenelzine, and it did produce significant improvement in a randomised, double blind placebo controlled trial,[50] while a trial of another MAO inhibitor, selegiline, showed some benefit which appeared unrelated to the antidepressant effect of monoamine oxidase inhibitors.[51]
Other antidepressants have also been investigated. Fluoxetine has most frequently been the drug of choice rather than tricyclic drugs, as it is said to have fewer sedative and autonomic nervous system effects. A Dutch study showed it to be ineffective on combating depressive symptoms found in ME, suggesting that these symptoms are different in origin from the depressive symptoms of endogenous depression.[52] A study comparing the effects of graded exercise and fluoxetine showed fluoxetine bringing improvements only in depressive symptoms and not in functional work capacity.[53] This illustrates the difficulty of comparing the results of different studies, in this case this latter study and the Dutch study referred to above, since much depends on the clinical case definition used to select patients with ME for the trial. A number of such definitions are in use, some of which exclude patients with depressive illness, while others do not. While fluoxetine may be ineffective, other antidepressants may not be. Thus an uncontrolled study of fluoxetine and buproprion showed some benefit from the use of buproprion in patients who did not respond to or could not tolerate fluoxetine.[54]
Another such study took as its initial hypothesis that ME involved defects of acetyl choline activity as a neurotransmitter in the brain, and investigated the effects of an acetylcholinesterase inhibitor designed to counteract these changes, galanthamine hydrobromide. Significant improvements were found in the treatment group in comparison with a placebo group, though the authors themselves urge caution in the interpretation of these results because of methodological difficulties with the trial.[55] It has been suggested that further large scale clinical trials be undertaken investigating other neurotransmitters, in particular serotonin.[56]
References
1. Jason LA, Richman JA, Rademaker AW et al. A community-based study of chronic fatigue syndrome. Archives of Internal Medicine 159(18): 2129-37. 1999.
2. Pagani M and Lucini D. Chronic fatigue syndrome: a hypothesis focusing on the autonomic nervous system. Clinical Science 96(1): 117-25. 1999.
3. Bakheit AM, Behan PO, Dinan TG et al. Possible upregulation of hypothalamic 5-hydroxytryptamine receptors in patients with postviral fatigue syndrome. BMJ 304: 1010-2. 1992.
4. Demitrack MA and Crofford LJ. Evidence for and pathophysiologic implications of hypothalamic-pituitary-adrenal axis dysregulation in fibromyalgia and chronic fatigue syndrome. Annals of the New York Academy of Sciences 840: 684-97. 1998.
5. Wakefield D, Lloyd A. Pathophysiology of myalgic encephalomyelitis. Lancet 2: 918-9. 1987.
6. Swanink CM, Melchers WJ, van der Meer JW et al. Enteroviruses and the chronic fatigue syndrome. Clinical Infectious Diseases 19(5): 860-4. 1994.
7. Peakman M. Deale A. Field R et al. Clinical improvement in chronic fatigue syndrome is not associated with lymphocyte subsets of function or activation. Clinical Immunology & Immunopathology 82(1): 83-91. 1997.
8. Wilson A. Hickie I. Lloyd A et al. Cell-mediated immune function and the outcome of chronic fatigue syndrome. International Journal of Immunopharmacology 17(8): 691-4. 1995.
9. Lerner AM, Zervos M, Dworkin HJ et al. New cardiomyopathy: Pilot study of intravenous ganciclovir in a subset of the chronic fatigue syndrome. Infectious Diseases in Clinical Practice 6(2): 110-117. 1997.
10. Hilgers A, Krueger GR et al. Postinfectious chronic fatigue syndrome: case history of thirty-five patients in Germany. In Vivo 5(3): 201-5. 1991.
11. Rowe KS. Double-blind randomized controlled trial to assess the efficacy of intravenous gammaglobulin for the management of chronic fatigue syndrome in adolescents. Journal of Psychiatric Research 31(1): 133-47. 1997.
12. Rowe KS. Five-year follow-up of young people with chronic fatigue syndrome following the double blind randomised controlled intravenous gammaglobulin trial. J Chronic Fatigue Syndr 5(3-4): 97-107. 1999.
13. Vollmer-Conna U, Hickie I, Hadzi-Pavlovic D et al. Intravenous immunoglobulin is ineffective in the treatment of patients with chronic fatigue syndrome. American Journal of Medicine 103(1): 38-43. 1997.
14. Lloyd AR, Hickie I, Brockman A et al. Immunologic and psychologic therapy for patients with chronic fatigue syndrome: a double-blind, placebo-controlled trial. American Journal of Medicine 94(2): 197-203. 1993.
15. Peterson PK. Shepard J. Macres M et al. A controlled trial of intravenous immunoglobulin G in chronic fatigue syndrome. American Journal of Medicine 89(5): 554-60, 1990.
16. Lloyd A, Hickie I, Wakefield D et al. A double-blind, placebo-controlled trial of intravenous immunoglobulin therapy in patients with chronic fatigue syndrome. American Journal of Medicine 89(5): 561-8. 1990.
17. Jiaxu C and Weiyi Y. Treatment of chronic fatigue syndrome with Chinese medicine. J Chronic Fatigue Syndrome 5(1): 61-65. 1999.
18. Andersson M, Bagby JR et al. Effects of staphylococcus toxoid vaccine on pain and fatigue in patients with fibromyalgia/chronic fatigue syndrome. European Journal of Pain 2(2): 133-142. 1998.
19. Pall ML. Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Medical Hypotheses 54(1): 115-125. 2000.
20. Werbach MR. Nutritional strategies for treating chronic fatigue syndrome. Alternative Medicine Review 5(2): 93-108. 2000.
21. Behan PO, Behan WM and Horrobin D. Effect of high doses of essential fatty acids on the postviral fatigue syndrome. Acta Neurologica Scandinavica 82(3): 209-16. 1990.
22. Warren G, McKendrick M and Peet M. The role of essential fatty acids in chronic fatigue syndrome. A case-controlled study of red-cell membrane essential fatty acids (EFA) and a placebo-controlled treatment study with high dose of EFA. Acta Neurologica Scandinavica 99(2): 112-6. 1999.
23. Cox IM. Campbell MJ. Dowson D. Red blood cell magnesium and chronic fatigue syndrome. Lancet 337: 757-60. 1991.
24. Hinds G, Bell NP et al. Normal red cell magnesium concentrations and magnesium loading tests in patients with chronic fatigue syndrome. Annals of Clinical Biochemistry 31(5): 459-61. 1994.
25. Seelig M. Review and hypothesis: Might patients with the chronic fatigue syndrome have latent tetany of magnesium deficiency. J Chronic Fatigue Syndrome 4(2): 77-108. 1998.
26. Plioplys AV and Plioplys S. Amantadine and L-carnitine treatment of Chronic Fatigue Syndrome. Neuropsychobiology 35(1): 16-23. 1997.
27. Stein DP, Dambrosia JM and Dalakas MC. A double-blind, placebo-controlled trial of amantadine for the treatment of fatigue in patients with the post-polio syndrome. Annals of the New York Academy of Sciences 753: 296-302. 1995.
28. Lund-Olesen LH. Lund-Olesen K. The etiology and possible treatment of chronic fatigue syndrome/ fibromyalgia. Medical Hypotheses 43(1): 55-8. 1994.
29. Ogawa M, Nishiura T, Yoshimura M et al. Decreased nitric oxide-mediated natural killer cell activation in chronic fatigue syndrome. European Journal of Clinical Investigation 28(11): 937-43. 1998.
30. Bralley JA. Lord RS. Treatment of chronic fatigue syndrome with specific amino acid supplementation. Journal of Applied Nutrition 46(3): (pp 74-78), 1994.
31. Martin RWY, Ogston SA and Evans JR. Effects of vitamin and mineral supplementation on symptoms associated with chronic fatigue syndrome with Coxsackie B antibodies. Journal of Nutritional Medicine 4(1): 11-23. 1994.
32. Kaslow JE, Rucker L and Onishi R. Liver extract-folic acid-cyanocobalamin vs placebo for chronic fatigue syndrome. Archives of Internal Medicine 149(11): 2501-3, 1989.
33. Morris DH and Stare FJ. Unproven diet therapies in the treatment of the chronic fatigue syndrome. Archives of Family Medicine 2(2): 181-6. 1993.
34. Ablashi DV. Levine PH. De Vinci C et al. Use of anti HHV-6 transfer factor for the treatment of two patients with chronic fatigue syndrome (CFS). Two case reports. Biotherapy 9(1-3): 81-6. 1996.
35. Levine PH. The use of transfer factors in chronic fatigue syndrome: prospects and problems. Biotherapy 9(1-3): 77-9. 1996.
36. De Vinci C. Levine PH. Pizza G. Fudenberg HH. Orens P. Pearson G. Viza D. Lessons from a pilot study of transfer factor in chronic fatigue syndrome. Biotherapy 9(1-3): 87-90. 1996.
37. Hana I, Vrubel J et al. The influence of age on transfer factor treatment of cellular immunodeficiency, chronic fatigue syndrome and/or chronic viral infections. Biotherapy 9(1-3): 91-5. 1996.
38. See DM. Tilles JG. alpha-Interferon treatment of patients with chronic fatigue syndrome. Immunological Investigations 25(1-2): 153-64. 1996.
39. Sudaholnik RJ, Reichenbach NL, Sobol RW et al. Biochemical defects in the 2-5A synthetase/RNase L pathway associated with chronic fatigue syndrome with encephalopathy. in Hyde BM and Goldstein J (editors). The Clinical and Scientific Basis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Nightingale Research Foundation. Ottawa. pp613-617. 1994.
40. Peterson DL, Strayer DR, Bastien S et al. Clinical improvements obtained with ampligen in patients with severe chronic fatigue syndrome and associated encephalopathy. in Hyde BM and Goldstein J (editors). The Clinical and Scientific Basis of Myalgic Encephalomyelitis/ Chronic Fatigue Syndrome Nightingale Research Foundation. Ottawa. pp634-638. 1994.
41. Cleare AJ, Heap E, Malhi GS et al. Low-dose hydrocortisone in chronic fatigue syndrome: a randomised crossover trial. Lancet 353: 455-8. 1999.
42. McKenzie R, O'Fallon A, Dale J et al. Low-dose hydrocortisone for treatment of chronic fatigue syndrome: a randomized controlled trial. JAMA 280(12): 1061-6. 1998.
43. Panay N and Studd JW. The psychotherapeutic effects of estrogens. Gynecological Endocrinology 12(5): 353-65. 1998.
44. Peterson PK. Pheley A. Schroeppel J et al. A preliminary placebo-controlled crossover trial of fludrocortisone for chronic fatigue syndrome. Archives of Internal Medicine 158(8): 908-14. 1998.
45. Himmel PB and Seligman TM. A pilot study employing Dehydroepiandrosterone (DHEA) in the treatment of chronic fatigue syndrome. J Clin Rheumatol 5(2): 56-59. 1999.
46. Kodama M, Kodama T and Murakami M. The value of the dehydroepiandrosterone-annexed vitamin C infusion treatment in the clinical control of chronic fatigue syndrome (CFS). I. A Pilot study of the new vitamin C infusion treatment with a volunteer CFS patient. In Vivo 10(6): 575-84. 1996.
47. Steinberg P, McNutt BE, Marshall P et al. Double-blind placebo-controlled study of the efficacy of oral terfenadine in the treatment of chronic fatigue syndrome. Journal of Allergy & Clinical Immunology 97(1): 119-26. 1996.
48. Moss RB, Mercandetti A and Vojdani A. TNF-alpha and chronic fatigue syndrome. Journal of Clinical Immunology 19(5): 314-6. 1999.
49. Dickinson CJ. Chronic fatigue syndrome - aetiological aspects. European Journal of Clinical Investigation 27(4): 257-67. 1997.
50. Natelson BH, Cheu J, Pareja J et al. Randomized, double blind, controlled placebo-phase in trial of low dose phenelzine in the chronic fatigue syndrome. Psychopharmacology 124(3): 226-30. 1996.
51. Natelson BH, Cheu J, Hill N et al. Single-blind, placebo phase-in trial of two escalating doses of selegiline in the chronic fatigue syndrome. Neuropsychobiology 37(3): 150-4. 1998.
52. Vercoulen JH, Swanink CM, Zitman FG et al. Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet 347: 858-61. 1996.
53. Wearden AJ, Morriss RK, Mullis R et al. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. British Journal of Psychiatry 172: 485-492.1998.
54. Goodnick PJ. Sandoval R et al. Bupropion treatment of fluoxetine-resistant chronic fatigue syndrome. Biological Psychiatry 32(9): 834-8, 1992.
55. Snorrason E, Geirsson A and Stefansson K. Trial of a selective acetylcholinesterase inhibitor, galanthamine hydrobromide, in the treatment of chronic fatigue syndrome. Journal of the Chronic Fatigue Syndrome 2(2-3): 35-54. 1996.
56. McCluskey DR. Pharmacological approaches to the therapy of chronic fatigue syndrome. Ciba Foundation Symposium. 173: 280-7. 1993.
To read Part II of this article pleae click HERE
Comments:
-
No Article Comments available