Positive Health Online
Your Country

Breast Cancer: Current Trends and Future Hopes
listed in cancer, originally published in issue 33 - October 1998
Introduction
Breast cancer is the third most common cancer in the world, creating a burden of disease comparable with that of colorectal cancer. With over half a million new cases in the world each year, only cancer of the lung and stomach occur with greater frequency, and breast cancer, overall, accounts for about 9% of cancer cases in the world, and over 18% of cancers occurring in women. It is most common in North America and western Europe, accounting for about one in four female cancers in these regions, while in the Far East (China and Japan), it is very much rarer. It is also common in southern South America. The highest incidence rates of all are found in Hawaii, where a rate of 93.9 per 100,000 female population has been reported, and in US white women. The incidence rises with age from about age 30, but more slowly after the menopause than before. There are ethnic variations, such as a high incidence in Israeli Jews compared with non-Jews in Israel. It is more common in single women, in higher social classes, and in urban rather than rural areas. About 1% of cases occur in males.[1]
The incidence of breast cancer has been increasing in most advanced industrialised countries throughout the post-war period. This is a result of increasing risks in successive birth cohorts, as studies, for example, from Scandinavia [2] and Singapore [3] have indicated. (See Table 1)
Table 1: Age-standardised incidence rates per 100,000 population (from cancer registries) (NOT SHOWN)
Mortality has increased less rapidly than incidence, but breast cancer is the most common cause of cancer deaths. It accounted for 18% of cancer deaths in 1995, [4] as shown in Figure 1 below.
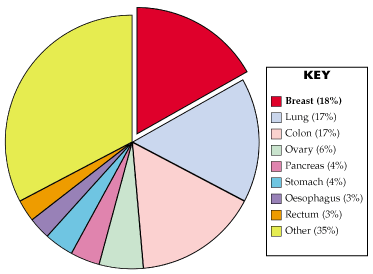
Figure 1: Causes of Cancer Mortality in Women, UK, 1995 (Source: Cancer Research Campaign)
Stage-specific survival has not greatly altered in recent years. This may indicate a higher proportion of early stage disease at diagnosis, perhaps attributable to screening programmes, since survival is very much better if the disease is detected and treatment initiated at an early stage. It is possible to quantify these stage-related differences in survival. The Thames Cancer Registry, for example, uses a simple classification of disease stage with four categories, viz:
I Localised tumour
II Tumour invading surrounding tissues
III Axillary lymph nodes involved
IV Distant metastases
Ten year survival for patients in category I was 64.9%, and for other patients 24.9%. Figure 2 shows stage-specific five-year survival for patients aged 15–74, diagnosed in 1986–89 in South East England.
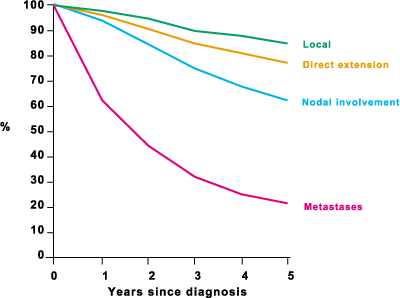
Figure 2: Stage-specific five-year relative survival for patients aged 15–17, diagnosed in 1986–89 in South East England
(Source: Cancer Research Campaign Fact Sheet 6. Breast Cancer – UK 1996)
The causal mechanisms underlying breast cancer have yet to be fully elucidated, but there is sufficient evidence to make it clear that environmental, social, behavioural and genetic factors are all involved. Migrant studies underline the importance of environmental factors, since rates found among migrants from low risk areas such as Japan to the United States tend to move towards the rates found among the rest of the population among whom they take up residence, within a relatively short period of time.[5] Diet may be an important factor, with associations of incidence with intake of fat, animal protein, and total calories,[6] but other studies have failed to confirm this. There have, in fact, been numerous case-control and prospective cohort studies which have considered the possible causal association between dietary fat and breast cancer, with conflicting results. However, it is actually very difficult to assess dietary fat, and to make comparisons between different populations, and there is no clear biological mechanism which could account for such a causal association.[7] Obesity is associated with increased risk in postmenopausal women.[8] There is an increased risk of breast cancer associated with the consumption of alcohol.[9] Ionising irradiation, whether among atomic bomb survivors or following multiple X-ray examinations, is associated with an increased risk of breast cancer, the risk increasing linearly with exposure.[10] There is strong evidence of a familial tendency. Sisters of people with bilateral premenopausal breast cancer are at increased risk,[11] and if the mother also has the disease the risk may reach 50%.[12]
The increase in incidence in the West in the post-war period is, in part, attributable to known risk factors such as smaller family size and increasing age at first pregnancy.[13] Combined oral contraceptives have been implicated as a risk factor in several studies, particularly for women who used such preparations early in their reproductive life, though there are difficulties in the interpretation of the data. [14] The age-specific incidence rate for breast cancer rises more slowly after the menopause. Early menopause, natural or induced, reduces the risk, while early menarche increases it. Exogenous oestrogens, such as HRT, may increase the risk. Low parity, and late ages at both first and last full-term pregnancies [15] appear to be independent risk factors. Conversely, early first birth and high parity appear to be independent protective factors. There may be a protective effect associated with breast feeding, but this requires further evaluation.
Variations in Treatment and Outcome
There are some important issues surrounding current patterns of treatment of breast cancer, and their impact on outcomes, which include survival, recurrence and quality of life.
The impact of referral delays on outcomes is a subject of concern. Thus, a study in British Columbia concluded that delay had a negative impact on long-term survival, and that this had not changed much over the years. It suggested that there was a considerable need for the improved education of doctors. [16] However, a Danish prospective nationwide cohort study, [17] looking at the prognostic impact of delay, distinguished between 'patient delay' and 'doctor delay'. The study found that patients with a long delay had larger tumours and more positive nodes than patients with a short delay, and that a long patient's delay was associated with an unfavourable outcome. On the contrary, the prognosis was superior for patients with a long doctor's delay, suggesting that doctors are able to distinguish between the less aggressive malignancies, where there is greater scope for effective medical intervention, and the more aggressive ones.
European and Canadian studies have tended to stress problems with the health care system as major causes of delay, but a study in Los Angeles [18] stressed the role of social factors such as socioeconomic status, ethnicity and age, and suggested that late referral was associated with social deprivation, but also that this effect appeared to be diminishing with time. Other studies, though, have failed to confirm this association.
Diagnostic and treatment methods vary between different types of hospitals. More investigations, and a wider range of treatments, tend to be available in teaching hospitals. [19] A study in Yorkshire of the period 1978 to 1992 found that there was a wide variation between districts in the treatments offered, and the systematic variation in mortality rates.[20] The mastectomy rate, and mastectomies as a proportion of all breast surgery, fell during the period, with a commensurate increase in breast-conserving surgery. Similar variations were noted in respect of the use of radiotherapy, hormonal therapy and adjuvant chemotherapy. A Scottish study, a little later on, found that the rate of surgery between 1987 and 1993 increased from 75% to 81% of cases, while breast conserving surgery (BCS) increased from 40% to 52% of all cases operated on. [21] It thus appears, therefore, that since the early 1980s there has been an increase in the proportion of patients receiving BCS. Similar increases have also occurred in the use of adjuvant radiotherapy and chemotherapy.
BCS is recommended by current consensus guidelines in early-stage disease, and a key issue is the extent to which it is actually practised. It has been clearly demonstrated that, in early-stage disease, there is little difference in survival following BCS or mastectomy. [22] In general, patients treated by BCS have survival rates at least as high as patients allocated to mastectomy. However, a survey of physicians in New England found the main determinants of surgical preference to be the patient's age, and the physician's attitude to patient participation in decision-making, while specialty, hospital size, and the availability of radiotherapy on site were important predictors of the use of adjuvant chemotherapy. [23] Such factors are important in causing geographical variations in patterns of treatment in North America, which a number of studies have detected. [24], [25]
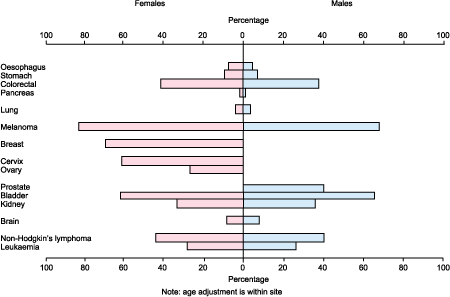
Figure 3: Five year relative survival (%), major cancer sites, England and Wales, 1989. (Source: Office for National Statistics)
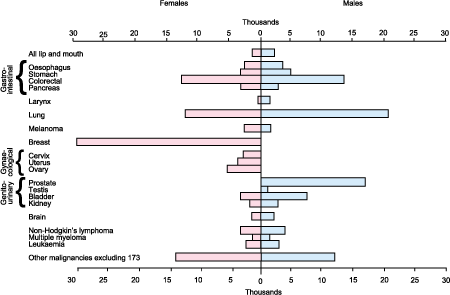
Figure 4: Registrations, major cancer sites, England and Wales, 1997 (estimated) (Source: Office for National Statistics)
Similar, though perhaps less marked variations are found in the rest of the world, including Europe and the United Kingdom. A questionnaire to surgeons in the mid-1980s, before the introduction of 'best practice' guidelines, indicated considerable diversity of practice. [26] Since the introduction of consensus guidelines, such heterogeneity has decreased, though not disappeared, with surgeons treating many cases being more likely to practise BCS.[27]
Evidence from Australia [28] and Italy [29] suggests that BCS is less frequently practised in rural than in urban communities, perhaps due to lack of access to post-operative radiotherapy. In the Netherlands, a study indicated smaller hospitals used fewer diagnostic tests and less conservative procedures than a comprehensive cancer centre. [30] A Norwegian study, though, found no differences between either different types of hospital in either treatment practices or outcomes, and attributed this to widespread adherence to treatment protocols. [31]
The overall picture which emerges regarding variations in approaches to the treatment of breast cancer is thus somewhat confused. Regional variation is well-documented, as well as variation at county and hospital level. BCS appears to be more frequently practised in teaching than in non-teaching hospitals. Age has been invoked as a factor in the decision to use BCS, with increased rates in younger women, but other studies have not confirmed this. Ethnicity has also been cited, but it has also been asserted that demography does not explain variations in BCS rates. Accessibility may be a factor, in that one study found the size of city and the availability of radiotherapy to be independent predictive factors of the use of BCS, but another study reached the opposite conclusion. Factors such as the stage (i.e. degree of progression) of the disease may explain a large part of the decision to use or not to use BCS, but another study found that treatment decisions bore little relationship to the stage of the tumour. Physicians' attitudes are clearly important as the decision process is largely doctor-driven, and older doctors may be less willing to embrace new approaches. There is evidence that the availability of consensus treatment guidelines and protocols may be effective in changing physicians' attitudes. Much of the evidence, though, is North American, and may largely reflect local conditions, so there is clearly a need for further research in the UK, if only to provide baseline data for enabling the impact of the Calman-Hine reorganisation of cancer services [32] to be evaluated.
Other aspects of good management practices are subject to similar types of variation, such as axillary node sampling, [21], [33] post-operative radiotherapy, [34] and the use of chemotherapy and tamoxifen. Thus studies in South East England have shown that chemotherapy was not routinely given to patients aged less than fifty with Stage II disease, contrary to consensus advice, [35] and that, while most patients over fifty (94%) received tamoxifen, very few (2.5%) received adjuvant chemotherapy. [36]
UK studies have shown marked differences in the quality of care in different hospital settings. In a comparison of a teaching hospital and a non-teaching hospital, [37] there were differences in practice, and survival was better at the teaching centre. The authors concluded that how women with breast cancer were treated was determined as much by where they are referred to as by scientific evidence. An audit study, in the North East Thames region, found significant associations between being a specialist centre and providing high quality care, but no differences were found between specialist (high volume) and non-specialist centres in factors important in survival. [38]
A major determinant of survival is the stage of the disease at the time of diagnosis. An American study found that survival at 10 years was 90% for Stage I disease, and 69% for Stage II. [39] Aspects of treatment also to some extent affect survival. Thus, in a study in Yorkshire, variation among consultants in treatment practices, and in the numbers of patients treated, accounted for 8% of survival differences, with the consultants treating large numbers of cases having the best survival rates. [40] This was in line with the findings of Richards and colleagues, who conducted a review and concluded that a substantial number of women receive sub-optimal care. They reported that cancer registry based studies had clearly demonstrated variations between surgeons and hospitals in the management of early breast cancer, and that the use, or not, of systemic adjuvant therapy did indeed influence outcome. [41]
Screening
Despite recent advances in understanding, too little is known about the pathogenesis of breast cancer for primary prevention to be feasible. Secondary prevention through screening offers an alternative which has been widely adopted. [42] Randomised controlled trials have demonstrated the efficacy of mass population screening, first in New York in 1963, [43] and then in Sweden. [44] The benefits in both cases were mostly to be found among women aged over fifty. It has been noted that very early stage cancers (ductal carcinomas in situ) are forming an increasing proportion of breast cancers, [45] and this undoubtedly reflects the impact of screening. In Britain, there has been a national breast cancer screening programme since 1988. [46] Early results suggested that quality targets were, by and large, being met, albeit with some local variations. [47], [48] The effect of establishing the programme has been to bring about an increase in the recorded incidence of breast cancer, but an ensuing decline in mortality among women aged 55+ has come too quickly to be attributable to the programme, and probably reflects the increased use of tamoxifen. [49] The increased number of cases ascertained is largely of early-stage lesions. [50]
The British programme targets women in the 50–64 age group, and allows for screening by mammography at three-year intervals, and there has been debate as to whether the optimum age group has been defined, and whether the right screening interval has been selected. There is evidence that reducing the screening interval to one year would enable comprehensive ascertainment of early stage disease in women aged over 64 [51] and in women in their forties, [52] though this would obviously have very considerable financial implications for the health care system. For women within the screening age group, there is also evidence that the three-year screening interval is too long. Thus a recent record linkage study in the North-West of England found that the incidence of 'interval' cancers (i.e. non-screen detected cancers in the screened population) in the third year after breast screening approaches that which would have been expected in the absence of screening. [53] Reducing the screening interval would clearly mean that many of these would become screen-detected at an earlier stage.
Future Possibilities
Some idea of likely developments in breast cancer treatment and outcomes over the next quarter of a century comes from a recent study from the Office of Health Economics, a research centre aligned with the pharmaceutical industry. The Association of the British Pharmaceutical Industry commissioned a study by the 'think-tank' the Battelle Institute to gauge 'the extent to which advances in biomedical technology and changes in health-related behaviour would affect the future health status of UK citizens', and to 'predict the number of cases and deaths from six major diseases (one of which was breast cancer) that may be avoidable'.[54]
The methodology used involved the calculation of a standard projection (SP), based on extrapolation for 25 years into the future of routinely available current data, assuming no further medical advances, and no changes in life patterns, over the period in question. Panels of experts for each disease were identified. Each panel member was asked to modify the standard projections in the light of his or expectations as to likely medical advances and behavioural changes, and the combined views of the experts were then used to produce a forecast projection (FP). Parallel studies were carried out in the UK, France and Germany. The results are summarised in Table 2.
Table 2: Expert projections as to likely changes in patterns of breast cancer over the next 25 years (NOT SHOWN)
There is clearly a problem with some of the base data used to prepare the standard projections, particularly for France. It should be borne in mind that neither France nor Germany had national comprehensive cancer registration schemes, so much of the incidence data would have had to be based on estimates. Nevertheless, what is interesting is that the experts in all three countries were unanimous in agreeing that changes of various types would result in modest reductions in incidence, and rather larger reductions in mortality. There was no consensus between countries, though, as to what would produce these changes, with the UK attaching most importance to behavioural change, while the French experts saw pharmaceutical advances as likely to contribute most, with the Germans occupying an intermediate position.
In the UK, it was likely that mortality and morbidity would increase by much less than the standard projection, and might even decrease, for a number of reasons. Firstly, in some parts of the country, notably the South-West, mortality was already beginning to decrease in the period 1984–1989. Secondly, the growth of screening programmes for early detection, and the likelihood of their extension to younger age groups, is resulting in a higher proportion of tumours being detected at an early stage, making them more amenable to treatment. Thirdly, relatively new drugs, in particular the anti-oestrogen tamoxifen, have proved to be effective in treating the disease. Fourthly, there are other new developments in treatment likely to become available in the next few years.
New health technologies at the level of cell biology and molecular biology create the prospect of gentler, less invasive and more effective treatments being developed. Immunotherapy and gene therapy in particular are currently very exciting areas of research, now that there is rapidly increasing knowledge of the roles of oncogenes and of loss of tumour suppressor genes in the pathogenesis and progress of cancer, and clinical trials are beginning to report positive conclusions. [55] Meanwhile, new approaches to the use of existing technology are continuing to reap dividends, for example in the use of high dose chemotherapy as a potentially curative treatment, either on its own [56] or in association with bone marrow transplantation. [57]
New pharmacological developments likely to contribute to reducing mortality and morbidity in the next 25 years include the use of monoclonal antibodies, which are likely to prove an effective means of treatment. Growth factors are an exciting research area, and the development of growth factor receptor blockers could have a significant impact on mortality. Interleukins have a wide range of effects, and their potential role in breast cancer has yet to be fully clarified. The use of interferons in breast cancer is theoretically interesting, but Phase I trials have been disappointing. Behavioural changes could contribute more, in particular participation in screening, stress reduction, and dietary education, especially to encourage low fat diets and the avoidance of excessive quantities of alcohol. New forms of chemotherapy, including chemosentisers, could make a major contribution to treatment.
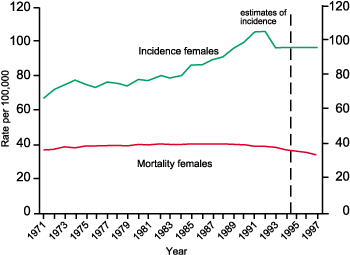
Figure 5 Breast Cancer: Directly age standardised rates. England and Wales 1971–1997 (Source: Office for National Statistics)
There is some scope for chemoprevention of breast cancer. Beta carotene is promising in this respect, but more especially for gastrointestinal tumours. Retinoids (isoretinoin and others) are experimental substances which act by being synergistic with interferon, and offer some prospect of being useful in the prevention of breast cancer. As regards the role of diet in the prevention of breast cancer, deficiencies of vitamins and micronutrients may be contributory factors, but it is not clear how important this is in Western societies, and the potential role that could be played by dietary anticarcinogens is also unclear.
Figure 8: One and five year survival of breast cancer cases diagnosed in 1989. (NOT SHOWN)
Complementary Therapy
Developments in the care of patients with breast cancer is not just a matter of developing and disseminating new technologies. Following the pioneering work of centres such as the Bristol Cancer Help Centre, there is an increasing awareness in the medical profession of the need for psychosocial support for patients, of the need for individualised care, of the crucial role the GP could exercise in providing this, and of the role of complementary therapies. [58] This underlines the need for better training of GPs in all aspects of supportive care, from psychosocial support to symptom control.
A prospective survey of patients attending a Complementary Cancer Therapy service concluded that complementary cancer therapy had a positive effect on psychological distress, anxiety, and quality of life, though it was not possible to determine which components of the therapy were most beneficial. [59] Similarly, an Italian study found that, while only about one in six breast cancer patients were using complementary therapies in addition to their conventional treatment, and they tended to be younger and more educated than non-users, levels of satisfaction were generally high. The most frequently used complementary therapies were homoeopathy, manual healing, herbalism and acupuncture, and they were more often used to alleviate physical distress than as a source of psychological support. [60]
Another study of breast cancer patients found that, while users and non-users of complementary therapies did not differ significantly in their beliefs about the causes of their breast cancer, there was a marked divergence in their beliefs about recovery. Users of complementary therapies tended to have beliefs of relatively recent origin, much influenced by their illnesses, unlike the non-users, whose beliefs were much longer term, or lifelong. [61]
There are studies which have shown that psychotherapy can be effective in modifying endocrine and immune function, which may have an impact on prognosis, [62] and it has been reported that psychosocial intervention in breast cancer can prolong survival time. [63] However, a great deal more work needs to be done to confirm these findings, as it very difficult to mount scientifically rigorous studies in this area, not least, because of the problems of selecting control groups in ways which are free of biases which could lead to distorted results.
Immunotherapy
Immunotherapy is a rapidly developing field with enormous implications for cancer therapy. It has been used with two main therapeutic objectives in mind. Firstly, the use of site-specific antibodies as a means of targeting chemotherapeutic substances and other materials directly at tumour cells, [64] and secondly by attempting to promote rejection of the tumour by the host, mainly through cell-mediated immunity system. There have been cases of breast cancer which have responded dramatically to this form of treatment. [65]
Monoclonal antibodies have been used to deliver to cancer cells immunotoxins which, in addition to a monoclonal antibody, contain a toxin such as a growth factor which proves lethal for the cancer cell. Such toxins may be of bacterial or plant origin, the latter including ricin, for example. [66] Another approach is to use a monoclonal antibody to block growth factor receptors and thereby retard cell progression, [67] while monoclonal antibodies have also been used to deliver combinations of interferon and tamoxifen to breast cancer cells. [68] The technique is steadily being refined. Thus the creation of a more efficient drug delivery vehicle in the form of a monoclonal antibody/liposome combination has been reported. [69]
Approaches to invoking cell-mediated immunity to treat breast cancer involve stimulating Natural Killer (NK) cells with thymostimulin to attack tumour cells, [70] administering interleukin, [71] interleukin producing cells [72] or lymphocytes. [73] These approaches have been used in a variety of clinical situations, and current research is focusing on reducing associated toxicity. [74] Some immunotherapeutic approaches make use both of targeted antibodies and cell therapy. Thus such antibodies can be used to deliver activated lymphocytes directly to tumour cells, [75] or indeed to activate such cells in the first place. [76]
Gene Therapy
Much progress in gene therapy has been made in the last few years. [77] Current research largely centres on the p53 tumour suppressor gene, both addressing the prognostic effect of alterations in this gene, [78] which are found in 55% of human cancers, [79] and developing ways to deliver the gene to tumour cells in order for it to exercise its suppressant effect.[80] Such delivery systems frequently involve adenoviruses. [81], [82], [83] The effect of reintroducing normal p53 to a cell containing ineffective mutant forms may be to initiate the process of apoptosis, or cell death. [84]
Cell Therapy
Cell therapy involves the modification of cell metabolism in order to inhibit proliferation of tumour cells. Topics being studied include the role of vitamin D3 analogues in targeting breast cancer cells, since they are known to have vitamin D3 receptors, [85] and the role of insulin-like growth factors (IGFs) in the growth of tumours and the possibility of blocking IGF receptors in cancer cells. [86] An important research topic at present is the role of telomerase. The telomeres are chromosomal elements involved in cell ageing. Cancer cells, which are immortal, contain high levels of telomerase, which destroys telomeres and hence arrests the normal ageing process of the cell, but telomerase levels appear to be reduced by chemotherapy. [87]
Conclusion
In an wide-ranging overview of this nature, it is impossible to go into a great deal of detail about the current state of knowledge and available treatments, and likely future developments, for a subject as complex as breast cancer. It should be clear, though, from this summary that the next few years hold out very exciting prospects for improvements in care and outcomes. Less invasive techniques will become much more effective and widely available. Treatment is likely to become much more individually-orientated, and, hand in hand with the development of gentler and less destructive therapies, is coming a greater understanding of the needs of the whole patient, and of the role which can and should be played in the overall process of patient care by complementary therapies.
References
1 Tomatis, L, Aitio, A, Day, NE, Heseltine, E, Kaldor, J, Miller, AB, Parkin, DM, Riboli, E (eds.) Cancer: Causes, Occurrence and Control, IARC Scientific Publications No. 100. Lyon, IARC, 1990, 69–72.
2 Hakulinen, T, Andersen, AA, Malker, B, Pukkala, E, Schon, G, Tulinius, H. Trends in cancer incidence in Nordic countries. Acta Pathol. Microbiol. Immunol. Scand. (Sect. A) (1986): 94 (Suppl. 288) 62–63.
3 Lee, HP, Day, NE, Shanmugaratnam, K (eds.). Trends in Cancer Incidence in Singapore 1968–1982. IARC Scientific Publications No. 91. Lyon, IARC, 1988.
4 Breast Cancer – UK (Factsheet 6.5). London, Cancer Research Campaign, 1996.
5 King, H, Li, JY, Locke, FB, Pollack, ES, Tu, JT. Patterns of site-specific displacement in cancer mortality among migrants: the Chinese in the United States. Am. J. Public Health (1985): 75 237–242.
6 Rohan, TE, Bain, CJ. Diet in the etiology of breast cancer. Epidemiol. Rev. (1987): 9 120–145.
7 Tomatis, L, et al (eds.) Cancer: Causes, Occurrence and Control, op.cit., 206–209.
8 Longnecker, MP, Berlin, JA, Orza, MJ, Chalmers, TC. A meta-analysis of alcohol consumption to risk of breast cancer. JAMA (1988): 260 252–256.
9 Paffenbarger, RS, Kampert, JB, Chang, HG. Characteristics that predict risk of breast cancer, before and after the menpause. Am. J. Epidemiol. (1980): 112 258–268.
10 Boice, JD, Land, CE, Shore, RE, Norman, JE, Tokunaga, M. Risk of breast cancer following low dose radiation exposure. Radiology (1979): 131 589–597.
11 Anderson, DE, Genetic study of breast cancer: identification of a high risk group. Cancer (1974): 34 1090–1097.
12 Schwartz, AG, King, MC, Belle, SH, Satariano, WA. Risk of breast cancer to relatives of young breast cancer patients. J. Natl. Cancer Inst. (1985): 75 665–668.
13 Blot, WJ, Devessa, SS, Fraumeni, JF. Declining breast cancer mortality among young Americam women. (1987): J. Natl. Cancer Inst. 78 451–454.
14 Tomatis, L, et al (eds.) Cancer: Causes, Occurrence and Control, op.cit., 152–153.
15 MacMahon, B, Cole, P, Lim, TM, Lowe, CR, et al. Age at first birth and cancer of the breast: a summary of an international study. Bull. World Health Organ. (1970): 43 209–221.
16 Elwood, M, Moorehead, WP. Delay in diagnosis and long-term survival in breast cancer. Brit. Med. J. (1980): 280 1291–1294.
17 Afzelius, P, Zedeler, K, Sommer, H, et al. Patient's and doctor's delay in primary breast cancer: prognostic implications. Acta Oncologica (1994): 33 (4):345–351.
18 Richardson, JL, Langholz, B, Bernstein, L, et al. Stage and delay in breast cancer diagnosis by race socioeconomic status age and year. Brit. J. Cancer (1992): 65 922–926.
19 Basnett, I, Gill, M, Tobias, J. Variations in breast cancer management between a teaching and a non-teaching district. Eur. J. Cancer (1992): 28A (12) 1945–1950.
20 Sainsbury, R, Rider, L, Smith, A, Macadam, A. Does it matter where you live? Treatment variation for breast cancer in Yorkshire. Brit. J. Cancer (1995): 71 (6) 275–1278.
21 Dewar, JA. Scottish breast cancer audit. British Journal Cancer (1997): S28 13.
22 Abrams, JS, Phillips, PH, Friedman, MA. Meeting highlights – a reappraisal of research results for the local treatment of early-stage breast cancer. J. Natl. Cancer Inst. (1995): 87 (24) 837–1845.
23 Liberati, A, Patterson, WB, Biener, L, McNeil, BJ. Determinants of physicians' preferences for alternative treatments in women with early breast cancer. Tumori (1987): 73 (6):601–609.
24 Farrow, DC, Hunt, WC, Samet, M. Geographic variation in the treatment of localized breast cancer. New England Journal of Medicine (1992): 326 (17):1097–1101.
25 Iscoe, NA, Goel, V, Wu, KY, et al. Variation in breast-cancer surgery in Ontario. Can. Med. Ass. J. (1994): 150 (3):345–352.
26 Morris, J, Royle, GT, Taylor, I. Changes in the surgical management of early breast cancer in England. Journal of the Royal Society of Medicine (1989): 82: 12–14.
27 McCarthy, M, Bore, J. Treatment of breast cancer in two teaching hospitals: a comparison with consensus guidelines. Eur. J. Cancer (1991): 27 (5): 579–582.
28 Craft, PS, Primrose, JG, Lindner, JA, McManus, PR. Surgical management of breast cancer in Australian women in 1993 – analysis of medicare statistics. Medical Journal of Australia (1997): 166 (12): 626–629.
29 GIVIO (Interdisciplinary Group for Cancer Care Evaluation). Survey of treatment of primary breast cancer in Italy. Brit. J. Cancer (1988): 57: 630–634.
30 Miransky, J, Kerner, JF, Sturgeon, SR, et al. A comparison of primary breast cancer management in small intermediate and large community hospitals and a comprehensive cancer center. Progress in Clinical & Biological Research (1984): 156: 87–96.
31 Norum, J. Breast conserving surgery in Northern Norway multicenter cooperative studies and treatment protocols ensure a high treatment standard. Int. J. Oncol. (1996): 8 (3) 633–637.
32 Department of Health/Welsh Office Expert Advisory Group. A Policy Framework for Commissioning Cancer Services (Calman/Hine Report), NHS Executive, 1995.
33 Richards, MA, Wolfe, CD, Tilling, K et al. Variations in the management and survival of women under 50 years with breast cancer in the South East Thames region. Brit. J. Cancer (1996): 73 751–757.
34 Goel, V, Olivotto, I, Hislop, TG, et al. Patterns of initial management of node-negative breast cancer in two Canadian provinces. Can. Med. Ass. J. (1997): 156 (1) 25–35.
35 Chouillet, AM, Bell, CMJ, Hiscox, JG. Management of breast cancer in southeast England. Brit. Med. J. (1994): 308 168–171.
36 Moritz, S, Bates, T, Henderson, SM, et al. Variation in management of small invasive breast cancers detected on screening in the former South East Thames region: observational study. Brit. Med. J. (1997): 315 1266–1272.
37 Basnett I, Gill M, Tobias J. Variations in breast cancer management between a teaching and a non-teaching district. Eur. J. Cancer (1992): 28A (12) 1945–1950.
38 Ma, M, Bell, J, Campbell, S, et al. Breast cancer management: is volume related to quality? Brit. J. Cancer (1997): 75 (11) 1652–1659.
39 Mansfield, CM, Komarnicky, LT, Schwartz, GF, et al. ten-year results in 1070 patients with stages I and II breast-cancer treated by conservative surgery and radiation therapy. Cancer (1995): 75 (9): 2328.
40 Sainsbury, R, Haward, B, Rider, L, et al. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet (1995): 345 1265–1270.
41 Richards, M, Sainsbury, R, Kerr, D. Inequalities in breast cancer care and outcome. Brit. J. Cancer (1997): 76 (5) 634–638.
42 Blamey, RW, Wilson,ARM, Patnick, J, Dixon, JM. Screening for breast cancer. Brit. Med. J. (1994): 309 1076–1079.
43 Shapiro, S, Venet, W, Strax, P, Venet, L. Current results of the breast cancer screening randomised trial: the Health Insurance Plan (HIP) of greater New York study. In Day, NE, Miller, AB (eds.), Screening for Breast Cancer. Toronto, Hans Huber,1988, 3–15.
44 Tabar, L, Fagerberg, G, Duffy, SW, Day, NE. The Swedish two county trial of mammographic screening for breast cancer: recent results and calculation of benefit. J. Epidemiol. Community Health. (1989): 43 107–114.
45 Fonseca, R, Hartmann, LC, Petersen, IA, Donohue, JH, et al. Ductal carcinoma in situ of the breast. Ann. Int. Med. (1997): 127 (11) 1013–1022.
46 Austoker, J. Screening and self-examination for breast cancer. Brit. Med. J. (1994): 309 (6948) 168–174.
47 Holland, PA, Dadra, S, Holt, S. The National Health Service Breast Screening Programme in the Trent region – Are we meeting the targets? Eur. J. Surg. Oncol. (1998): 24 (2) 99–103.
48 Chamberlain, J, Moss, SM, Kirkpatrick, AE, Michell, M, Johns, L. National Health Service breast screening programme results for 1991–2. Brit. Med. J. (1993): 307 353–356.
49 Quinn, M, Allen, E. Changes in incidence of and mortality from breast cancer in England and Wales since introduction of screening. Brit. Med. J. (1995): 311 1391–1395.
50 Lindgren, A, Holmberg, L, Thurfjell, E. The influence of mammography screening on the pathological panorama of breast cancer. APMIS. (1997): 105 (1) 62–70.
51 Field, LR, Wilson, TE, Strawderman, M, et al. Mammographic screening in women more than 64 years old: A comparison of 1- and 2-year intervals. Am. J. Roentgenology. (1998): 170 (4) 961–965.
52 Bjurstam, N, Bjorneld, L, Duffy, SW, Smith, TC, et al. The Gothenburg breast screening trial: First results on mortality, incidence, and mode of detection for women ages 39–49 years at randomization. Cancer. (1997): 80 (11) 2091–2099.
53 Woodman, CBJ, Threlfall, A, Boggis, CRM, Prior P. Is the three year breast screening interval too long? Occurrence of interval cancers in NHS breast screening programme's north western region. Brit. Med. J. (1995): 310 224–226.
54 The Impact of Behavioural and Biomedical Advance on Health Trends over the next 25 Years (OHE Briefing no. 31). London, Office of Health Economics, November 1994.
55 Gordon, A. The increasing efficacy of breast cancer treatment. Clin. Oncol. (1997): 9 (5) 338–342.
56 Bezwoda, WR. High-dose chemotherapy with hematopoietic rescue in breast cancer: From theory to practice. Cancer Chemotherapy & Pharmacology (1997): 40 (Suppl.) S79–S87.
57 Vahdat, L, Antman, K. High dose chemotherapy with autologous stem cell support for breast cancer. Current Opinion in Hematology (1997): 4 (6) 381–389.
58 Ashby MA, Kissane DW, Beadle GF, Rodger A. Psychosocial support, treatment of metastatic disease and palliative care. Med. J. Australia. (1996): 164 (1) 43–45,47–49.
59 Clover A, Last P, Fisher P, Wright S, Boyle H. Complementary cancer therapy: A pilot study of patients, therapies and quality of life. Complementary Therapies in Medicine (1995): 3 (3) 129–133.
60 Crocetti E, Crotti N, Feltrin A, Ponton P, et al. The use of complementary therapies by breast cancer patients attending conventional treatment. Eur. J. Cancer (1988): 34 (3) 324–328.
61 Brown PJ, Carney PA. Health beliefs and alternative medicine: A qualitative study of breast cancer patients. J. Cancer Education (1996): 11 (4) 226–229.
62 Van der Pompe G, Duivenvoorden HJ, Antoni MH, Visser A, Heijnen CJ. Effectiveness of a short-term group psychotherapy program on endocrine and immune function in breast cancer patients: An exploratory study. J. Psychosomatic Res. (1997): 42 (5) 453–466), 1997.
63 Kogon MM, Biswas A, Pearl D, Carlson RW, Spiegel D. Effects of medical and psychotherapeutic treatment on the survival of women with metastatic breast carcinoma. Cancer. (1997): 80 (2) 225–230.
64 Scott AM, Welt S. Antibody-based immunological therapies. Current Opinion in Immunology (1997): 9 (5) 717–722.
65 Skeel RT, Quan WDY, Palackdharry CS. Common cancers – Immunotherapy and multidisciplinary therapy: Parts III and IV. Disease-A-Month (1997): 43 (11) 749–808.
66 Kreitman RJ, Pastan I. Immunotoxins for targeted cancer therapy. Advanced Drug Delivery Reviews (1998): 31 (1–2) 53–88.
67 Mendelsohn J. Epidermal growth factor receptor inhibition by a monoclonal antibody as anticancer therapy. Clinical Cancer Research. (1997): 3 (12 II) 2703–2707.
68 Lindner DJ, Borden EC. Synergistic antitumor effects of a combination of interferon and tamoxifen on estrogen receptor-positive and receptor-negative human tumor cell lines in vivo and in vitro. Journal of Interferon & Cytokine Research. (1997): 17 (11) 681–693.
69 Park JW, Hong K, Kirpotin DB, Meyer O, et al. Anti-HER2 immunoliposomes for targeted therapy of human tumors. Cancer Letters (1997): 118 (2) 153–160.
70 Meneses G, Delgado MA, Perez-Machado MA, Prieto A, et al. Thymostimulin increases natural cytotoxic activity in patients with breast cancer. Int. J. Immunopharmacol. (1997): 19 (4) 187–193.
71 Everse LA, Renes IB, Jurgenliemk-Schulz IM, Rutgers DH, et al. Local low-dose interleukin-2 induces systemic immunity when combined with radiotherapy of cancer. A pre-clinical study. Int. J. Cancer (1997): 72 (6) 1003–1007.
72 Lotze MT, Hellerstedt B, Stolinski L. Tueting T, et al. The role of interleukin-2, interleukin-12, and dendritic cells in cancer therapy. Cancer Journal From Scientific American (1997): 3 (suppl. 1) S109–S114.
73 Or R, Ackerstein A, Nagler A, Kapelushnik J, et al. Allogeneic cell-mediated immunotherapy for breast cancer after autologous stem cell transplantation: A clinical pilot study. Cytokines, Cellular and Molecular Therapy (1998): 4 (1) 1–6.
74 Ballen K, Stewart FM. Adoptive immunotherapy. Current Opinion in Oncology (1997): 9 (6) 579–583.
75 Coulie PG. Human tumour antigens recognized by T cells: New perspectives for anti-cancer vaccines? Molecular Medicine Today (1997): 3 (6) 261–268.
76 Curnow RT. Clinical experience with CD64-directed immunotherapy. An overview. Cancer Immunology, Immunotherapy (1997): 45 (3–4) 210–215.
77 Schmidt-Wolf GD, Schmidt-Wolf IGH. Bone marrow and clinical gene therapy. J. Hematotherapy (1995): 4 (6) 551–561.
78 Schmutzler RK, Bierhoff E, Werkhausen T, Fimmers R, et al. Genomic deletions in the BRCA1, BRCA2 and TP53 regions associate with low expression of the estrogen receptor in sporadic breast carcinoma. Int. J. Cancer (1997): 74 (3) 322–325.
79 Lopez-Guerrero JA, Gilabert PB, Gonzalez EB. Biological and oncological significance of the molecular alterations of p53 in human cancer. Oncologia (1997): 20 (12) 25–38.
80 Walther W, Wendt J, Stein U. Employment of the mdr1 promotor for the chemotherapy-inducible expression of therapeutic genes in cancer gene therapy. Gene Therapy. (1997): 4 (6) 544–552.
81 Putzer BM, Bramson JL, Addison CL, Hitt M, et al. Combination therapy with interleukin-2 and wild-type p53 expressed by adenoviral vectors potentiates tumor regression in a murine model of breast cancer. Human Gene Therapy (1998) 9 (5) 707–718.
82 Nielsen LL, Gurnani M, Syed J, Dell J, et al. Recombinant E1-deleted adenovirus-mediated gene therapy for cancer: Efficacy studies with p53 tumor suppressor gene and liver histology in tumor xenograft models. Human Gene Therapy (1998): 9 (5) 681–694.
83 Le XF, Vallian S, Mu ZM, Hung MC. Chang K-S. Recombinant PML adenovirus suppresses growth and tumorigenicity of human breast cancer cells by inducing G1 cell cycle arrest and apoptosis. Oncogene (1998): 16 (14) 1839–1849.
84 Li P, Bui T, Gray D, Klamut HJ. Therapeutic potential of recombinant p53 overexpression in breast cancer cells expressing endogenous wild-type p53. Breast Cancer Research & Treatment. (1998): 48 (3) 273–286.
85 Koike M, Eistner E, Campbell MJ, Asou H, et al. 19-nor-hexafluoride analogue of vitamin D3: A novel class of potent inhibitors of proliferation of human breast cell lines. Cancer Res. (1997): 57 (20) 4545–4550.
86 Neuenschwander S, Roberts CT, LeRoith D. Growth inhibition of MCF-7 breast cancer cells by stable expression of an insulin-like growth factor I receptor antisense ribonucleic acid. Endocrinology (1995): 136 (10) 4298–4303.
87 Hoos A, Hepp HH, Kaul S, et al. Telomerase activity correlates with tumor aggressiveness and reflects therapy effect in breast cancer. Int. J. Cancer (1998): 79 (1) 8–12.
Comments:
-
No Article Comments available