Positive Health Online
Your Country

Letters to the Editor Issue 232
listed in letters to the editor, originally published in issue 232 - August 2016
Will EFSA Remove Road Blocks for Nutrients in Supplements?
by Dr Rob Verkerk - Alliance for Natural Health International
For those who’ve watched closely the issue of EU regulation of nutrients over the last decade and a half, you’ll recall that dozens of vitamin and mineral sources were effectively banned as food supplements by the passage of the EU Food Supplements Directive back in 2002. The reason? They didn’t make their way onto a positive list of permitted nutrients. In true, ‘boil the frog slowly’ EU style, it took a further 8 years for the ban to be implemented. Among the nutrients that disappeared were all forms of vanadium and silver, the majority of forms of boron and big slather of many mineral amino acid chelates including the forms used as intermediates most directly by the Krebs cycle in our all-important energy-producing organelles, our mitochondria.
Saving Natural Sources from the EU Abyss
By taking a case to the High Court in London and then to the European Court of Justice, we established acceptance by the European Commission, but not until 2007, that natural sources of vitamins and minerals were outside of the scope of this EU law. In the process, these natural sources of ingredients were rescued from the EU’s abyss, and that’s very good both for freedom of choice and for public health, given these types of nutrients are increasingly being demonstrated as among the most beneficial as they are derived directly from food sources. Examples of these ‘rescued’ vitamins and minerals include B vitamins from meat sources, and various forms of calcium, magnesium or iodine from seaweeds.
EU Obstacles to Freedom of Choice
While the European Commission has always upheld it didn’t impose any bans, what it did do is present an obstacle to additional nutrients being admitted to the positive list of permitted nutrients that effectively acted as a barrier, especially to smaller companies with less resources. The obstacle has existed since 2001 as a guidance document that provides information on the data requirements for any company wanting to have a new vitamin or mineral form added to the positive list. The guidance was produced not by the European Food Safety Authority (EFSA) which evaluates the dossiers, but by its predecessor, the Scientific Committee on Food (SCF) of the European Commission.
It’s therefore wholly appropriate EFSA puts out a public consultation asking for inputs from interested parties on how a revision to the guidance should be made. Whether it listens to concerns is of course another matter. The deadline for submissions was 20 July 2016; ANH-Intl has made a submission. It was a perfect opportunity for ANH-Intl to make EFSA again aware of the relevance of the work in which we commissioned the Dutch research organisation, TNO, to develop a new risk/benefit model for micronutrients.
Trying to Dismantle Barriers to Freedom of Choice
Among the key points which we have raised in our consultation response are the following:
- The safety of the intact source must take into account, where appropriate, the food matrix with which the nutrient might be consumed. If an ingredient can be made safe by consuming it with food, the maximum permitted dose of the nutrient should not be limited to a dose that might give rise to an adverse reaction if the ingredient was consumed on an empty stomach;
- Bioavailability should be part of the safety assessment, not a separate part. In our own case in the European Court of Justice, the court ruled that the only factors relevant to assessing whether or not a nutrient should be available for sale were its safety and bioavailability. The minimum requirements should be clarified for each of these, and these procedures should not be in any way onerous as that would pose unnecessary restrictions on trade and consumer freedoms;
- There should be no requirement for animal studies. Where adequate human data are available, these are all the data that should be required. Animal data are not only less reliable than human data given physiological differences between animal species and humans and associated translational difficulties, animal studies are increasingly viewed as unethical and wasteful;
- There should be an opportunity to provide data on the risk/benefit profile of the nutrient, where applicable. This is because without consideration of benefit, EFSA evaluations end up centring on the most sensitive risk factor among the most sensitive groups of people, this being a major feature which we exposed in our 2010 Toxicology papers, as follows:
- Verkerk RH, Hickey S. A critique of prevailing approaches to nutrient risk analysis pertaining to food supplements with specific reference to the European Union. Toxicology. 2010 Nov 28;278(1):17-26. doi: 10.1016/j.tox.2009.12.017. Epub 2009 Dec 23. Review.
- Verkerk RH. The paradox of overlapping micronutrient risks and benefits obligates risk/benefit analysis. Toxicology. 2010 Nov 28;278(1):27-38. doi: 10.1016/j.tox.2010.02.011. Epub 2010 Feb 24. Review.
We have therefore drawn EFSA’s attention (again) to the very recently published ‘ahead of print’ paper on risk/benefit assessment of micronutrients by TNO. The reference to the paper is as follows:
- Krul L, Kremer BH, Luijckx NB, Leeman WR. Quantifiable risk-benefit assessment of micronutrients: from theory to practice. Crit Rev Food Sci Nutr. 2016 May 17:0. [Epub ahead of print]
The full paper is available for download.
We will keep you appraised as to how EFSA responds - and whether its revised guidance removes - or possibly imposes even greater - barriers to freedom of choice.
Further Information
Republished from Alliance for Natural Health ANH International
Rob Verkerk | ANH Intl: info@anhinternational.org
Brexit - A Lucky Escape from the Vitamin Laws
by Lynne Mctaggart
Whether or not you’re for or against Brexit, there’s one undeniable area where Britain has an opportunity to improve things: the natural health and supplements market.
Back in 2000, the Brussels European Commission declared that there was a wide disparity between the laws of individual EU members on the dosages allowed for vitamin supplements.
In France and Germany, for instance, no products containing more than one to three times the RDA could be sold without a pharmaceutical license, while the UK and the Netherlands had always enjoyed liberal laws relating to the content of vitamins, with few trade restrictions across other European countries.
The effect of this was that, in certain countries of Europe, local suppliers had to stand by watching helplessly, their hands tied by their own stringent laws, while British exporters declare open season on their local vitamin business. It was amid such a climate of obvious trade disparity that the EU suggested a directive which would standardize laws concerning vitamin supplements all across Europe and protect consumers. The EU also wished to create a standardized market for health foods and supplements under the guise of setting a universal standard of safety. So far, so good.
In the Hands of Giants
Although painted with a consumerist face, these directives were patently about creating a level commercial playing field. They would allow the big vitamin giants to sell their products across Europe without having to reformulate them to comply with the requirements of individual countries. This meant that cheap low-grade products could be produced in bulk quantities at even cheaper cost and with greater profits.
The directive sought to create a single market for food supplements through several means, including devising a list of ingredients which may be used in supplements, creating common maximum permitted levels of these nutrients, providing common criteria for purity and labelling, and requiring that all member states prohibit any trade in products not complying with the directive.
One of the main issues of the directive centred around the use of a 'positive list' of permitted ingredients. At the end of the directive, a list is attached of nutrients which are approved for inclusion in food supplements. According to Dr Rob Verkerk, Director of the Alliance for Natural Health and an internationally acclaimed expert on the effect of the EU laws on the natural products industry, the end result of this directive has “prevented legal sale of many hundreds of mineral forms, including all forms of silver and vanadium.”
The Positive List
But the most worrying portion of the directive concerned the criteria for determining permitted levels of nutrients - still to be decided.
In making their determination, the European Commission indicated that it will take into account the highest intake shown to be safe before adverse events appear, plus the intake of nutrients from a 'normal' diet, plus the intake from 'fortified foods'. They've also indicated that they will subtract some amounts just to be on the safe side.
This means in practice is that the levels will be set well below levels demonstrated to be therapeutically effective.
For instance, what may finally emerge is a level of B6 as low as 5 mg. This would mean that British women taking 50-100 mg of B6 for PMS per day would have to swallow 10 to 20 vitamin pills every day just to maintain the levels they are used to.
There are 11 more directives like this, which include:
- The Human Medicinal Products Directive. This gave the EU a legal framework to classify any product as ‘medicinal’ (and therefore a drug) if there is any evidence that it actually helps anyone - and therefore be subject to the same sort of massively expensive testing and proof required of drugs;
- The Traditional Herbal Directive, which has cleared off the shelves of health stores any traditional herbal remedy without at least 15 years of safe use in the EU and some with a mix of herbs;
- The Nutrition and Health Claims Regulation, which has banned more than 2000 health claims with extremely plausible evidence, and so has essentially prevented the health industry from telling consumers that their products have any benefit whatsoever;
- The Food Information for Consumers Regulation, which clearly encourages consumers to opt for highly processed foods because they will have more labelling information than natural foods;
- The Pesticides Residue Regulation. This insidious law both mandates the use of the known carcinogenic pesticide glyphosate and ignores the combined effects of multiple pesticides.
As Rob Verkerk wrote recently, the EU’s “convoluted law-making process commission often means that you start with laws that are totally justifiable” but then “the laws get pulled, pushed, squeezed and distorted by a plethora of different interests over the lengthy time they are processed in the EU’s law-making sausage factory.”
Above all, he says, the law-making process “is stage-managed by the European Commission that is well known for running rough-shod over the last remnants of the democratic process. Compromise packages are the name of the game, and they typically involve back-room deals between Commissioners, rapporteurs and whichever Big Business sector has made the best case for why its interests need protecting over and above any public health interests or fundamental public freedoms.”
All of these laws reveal the heavy hand of the Big Pharma and Big Food industry lobbies to a shameful degree. One of the first tasks of the new British government will be to extricate itself from these overly restrictive laws and revitalize the innovative health industry. This will involve disbanding the positive list, studying the huge evidence of the benefits of high-dose supplements, relaxing some of the restrictions on herbal medicine, and disassociating Britain’s health industry from many of the restrictive definitions that essentially ban many products with an enormous amount of evidence.
Now is the time for organizations in the natural industry and all interested consumers to shout loudly about reform. If you want to have full access to natural health products, share this blog, write about this widely and encourage your British friends to write to their MP.
At last the British government is able to listen.
Further Information
Republished from Lynne McTaggart’s Blog – Brexit a Lucky Escape from the Vitamin Laws
Lynne McTaggart email@wddty-publishing.ccemails.com
lynnemctaggart.com
Viruses Turbo-Charge Bacterial Evolution in Cystic Fibrosis Infections
Scientists in the UK have found new evidence that tiny viruses called bacteriophages turbo-charge the evolution of bacteria that cause lung infections in Cystic Fibrosis patients. And they say because antibiotics can activate bacteriophages, certain medical treatments therapies might make the infections even more difficult to treat.
Cystic Fibrosis (CF) patients suffer from life-long lung infections caused by the environmental bacterium Pseudomonas aeruginosa. These infections worsen patient health and limit life-expectancy. Over the years of the infection, the bacteria evolve to become better adapted to the lung environment, becoming very difficult to treat. Scientists in the UK have found new evidence that viruses infecting the bacteria, called bacteriophages, can speed-up this bacterial evolution.
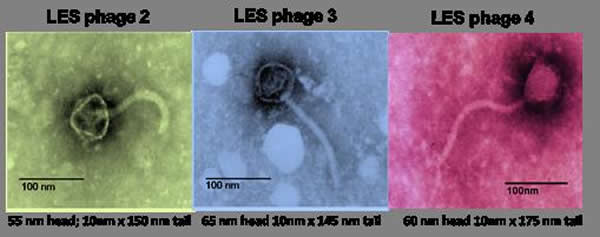
A team of scientists from the Universities of Liverpool, Salford and York evolved populations of the bacterium P. aeruginosa with or without bacteriophages in a growth medium designed to replicate the sputum in CF lungs. They then tracked evolutionary change in the bacterium using genome sequencing. The study, published in the Proceedings of the National Academy of Sciences, reports that bacteria with bacteriophages evolved faster to adapt to life in sputum. This happened because the bacteriophage jumped into the bacterium’s DNA, increasing the number of useful mutations that natural selection could use.
Many of the mutations seen in the lab-evolved bacteria, including those caused by the bacteriophage, are also commonly seen in bacteria isolated from CF infections. Because bacteriophages live wherever you find bacteria, including in the lungs of CF patients, this could mean that they play an important role in bacterial evolution in the clinic.
Dr Chloe James, lecturer in Medical Microbiology at the University of Salford, said: “We knew from our previous work that the bacteriophages infecting P. aeruginosa were commonly found in CF patient sputum. We now know that these bacteriophages can speed up evolution helping bacteria adapt to living in a sputum-like environment.”
Prof Craig Winstanley, of the University of Liverpool, said: “Because some antibiotics can activate bacteriophages, some antibiotic therapies might help bacteria to adapt to the lungs faster making the infections even more difficult to treat.”
Prof Michael Brockhurst, of the University of York, added: “To design better treatments and preserve our antibiotics we urgently need to better understand how bacteria evolve in infections. These new results suggest that bacteriophages may play a much bigger role than previously thought, by turbo-charging evolutionary adaptation.”
Notes
Temperate phages both mediate and drive adaptive evolution in pathogen biofilms was published on July 4, 2016 in the Proceedings of the National Academy of Sciences, and authored by Emily Davies,[1,2[ Chloe James,[1,3] David Williams,[1,2] Siobhan O’Brien,[4] Joanne Fothergill,[1] Sam Haldenby,[2[ Steve Paterson,[2] Craig Winstanley,[1] Michael A. Brockhurst.[4]
1. Institute of Infection and Global Health, University of Liverpool, Liverpool, UK.
2. Institute of Integrative Biology, University of Liverpool, Liverpool, UK.
3. School of Environment and Life Sciences, University of Salford, Manchester, UK.
4. Department of Biology, University of York, York, UK.
Further Information
PNAS provides journalists with access to embargoed manuscripts through EurekAlert!. Journalists should register with EurekAlert! at http://www.eurekalert.org/register.php and request access to PNAS materials. If they are already registered with EurekAlert!, they can request access to PNAS at http://www.eurekalert.org/account.php.
For more about Cystic Fibrosis, go to https://statistics.blf.org.uk/cystic-fibrosis or https://www.cysticfibrosis.org.uk /
Source
Hollyman Gareth <G.B.Hollyman@salford.ac.uk>
www.salford.ac.uk/news/articles/gareth/viruses-turbo-charge-bacterial-evolution-in-cystic-fibrosis-infections
Comments:
-
No Article Comments available