Positive Health Online
Your Country

Letters to the Editor Issue 243
listed in letters to the editor, originally published in issue 243 - January 2018
Significant Breakthrough Toward new Superbug-Killing Antibiotic Teixobactin
Scientists working to develop a ‘game-changing’ new antibiotic have made a significant advance towards creating commercially viable drug treatments by producing two simplified synthetic versions of the substance which are just as potent at killing superbugs like MRSA as its natural form. The breakthrough by researchers at the University of Lincoln, UK, marks another important step to realizing the potential of teixobactin in aiding the global fight against antibiotic-resistant pathogens. Teixobactin is a recently discovered natural antibiotic which many in the international scientific community believe could lead to creation of the first commercially viable new antibiotic drug in 30 years.
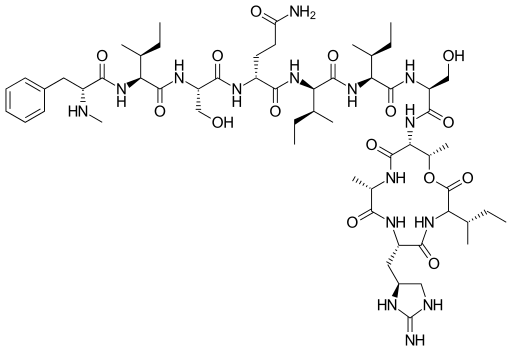
Structure of Teixobactin
Source: https://en.wikipedia.org/wiki/File:Teixobactin.svg
This image is in the public domain; it consists entirely of information
that is common property and contains no original authorship.
The Lincoln team has successfully synthesized new simplified versions of teixobactin which harness the same powerful antibiotic effects in a way that could be produced on a commercial scale. Their findings are published in the Royal Society of Chemistry's journal, Chemical Science.[1]
Dr Ishwar Singh has taken a significant step towards developing antibiotics capable of killing superbugs after successfully producing two simplified synthetic versions of teixobactin which are just as potent as its natural form and which use readily available amino acids – something which had been a major stumbling block for the scientific community’s ability to develop a commercially viable drug which could benefit patients until now.
He’s done this by contradicting current scientific understanding about the type of amino acid which can be swapped out with ones in the antibiotic’s structure, and replacing them with ones that do not have a side chain to ‘bind’ to the bacterial target; the results are as potent as the real thing, something which previous synthesized versions did not achieve. This could lead to the first new antibiotics in around 30 years. Readers may be interested in a timely exhibition about superbugs currently taking place British Science Museum’s exhibition.
Until now, scientists attempting to synthesise teixobactin believed they needed to use cationic (or positively charged) amino acids which bind to the bacterial target using a ‘side chain’. This meant they had to use either the very rare amino acid found naturally in teixobactin, called enduracididine, or alternative ones which had lower potency against superbugs.
Each amino acid sits at a specific place in teixobactin’s structure; the Lincoln team has now successfully replaced enduracididine – which holds position ten – with two alternative amino acids which are not positively charged. These amino acids lack the ‘binding’ part, over-turning the prior understanding that enduracididine is essential for to so-called ‘target binding’ to be highly potent against superbugs.
With this new knowledge, synthesized versions of teixobactin can be more easily developed, taking the process from up to 30 hours to just ten minutes for a single coupling step – a significant step towards turning teixobactin into a viable new drug. Importantly, the two new simplified forms of teixobactin have also proven to have identical potency against superbugs as the natural form of teixobactin.
Dr Ishwar Singh, a specialist in novel drug design and development from the University of Lincoln’s School of Pharmacy, is leading the research team. He explained: “When teixobactin was discovered it was ground breaking in itself as a new antibiotic which kills bacteria without detectable resistance including superbugs such as MRSA. We have been investigating a way to simplify the design while retaining the high potency against resistant bacteria such as MRSA.
“This simplified design and more efficient synthesise will enable work to be carried out at a commercial level. Enduracididine was severely limiting our ability to do this because of its scarcity, a complex multistep synthesis, and long and repetitive steps of between 16 and 30 hours with high failure rate and very low yields.
“We needed to make a change to the structure so that we could make the molecule more viable for drug development. We had tried replacing it with other amino acids with a similar make up, but they all were less potent in comparison to the natural form of teixobactin. Now, we have discovered that we can in fact use amino acids which are structurally different, and are commercially-available. They are also 16 times more potent than a clinically-used antibiotic in killing the superbug MRSA, and they were also highly potent against other antibiotic-resistant infections, such as vancomycin resistant enterococci, and tuberculosis.”
The work builds on the success of the team’s pioneering research to tackle antimicrobial resistance over the past 18 months. Dr Singh is working with colleagues from the School of Life Sciences and the School of Chemistry at the University of Lincoln to develop teixobactins into a viable drug.
It has been predicted that by 2050 an additional 10 million people will succumb to drug resistant infections each year. The development of new antibiotics which can be used as a last resort when other drugs are ineffective is therefore a crucial area of study for healthcare researchers around the world.
Source and Further Information
Cerri Evans PR Officer, Tel: +44 (0)1522 886165, Cerri Evans CEvans@lincoln.ac.uk
Reference
1. A. Parmar, A. Iyer, S. H. Prior, D. G. Lloyd, E. Goh, C. Vincent, T. Palmai-Pallag, C. Bachrati, E. Breukink, A. Madder, R. Lakshminarayanan, E. J. Taylor and I. Singh, Teixobactin analogues reveal enduracididine to be non-essential for highly potent antibacterial activity and lipid II binding Chem. Sci., 2017, DOI: 10.1039/C7SC03241B.
http://pubs.rsc.org/en/content/articlelanding/2017/sc/c7sc03241b#!divAbstract
Most New ‘Breakthrough’ Cancer Drugs Don’t Work
While the USA represents the single biggest customer for the pharmaceutical industry, Europe has long been the nerve centre for R&D of the global industry, with Swiss (Novartis, Roche), French (Sanofi), UK (GlaxoSmithKline, AstraZeneca) and German (Bayer, Boehringer Ingelheim, Merck) companies dominating.
Cancer drugs have a greater share of the global market than any other drug category. Of these, chemotherapy drugs contribute more to sales than any other category. What’s more, there’s been a greater-than-predicted surge in more targeted drugs that are promoted as being more effective than the older, versions they replace.
Assuming these new drugs work, can be afforded (they are very expensive) and don’t cause more harm than good, all is well. But that’s where the fairy story falls apart, as revealed in a study by a research team headed by King’s College London scientist Dr Courtney Davis and published in the British Medical Journal on 5 October (2017).
The big take home from this study was that the majority of new cancer drugs being licensed by the European drugs regulator, the European Medicines Agency (EMA), have not been demonstrated to work. That’s when important things like cancer survival rates over 5 years and quality of life improvements are evaluated.
This discovery, in essence, drives a coach and horses through the entire edifice of drug regulation that we’re told repeatedly exists to ensure that licensed drugs are proven effective. Not so, it seems, and this issue isn’t just confined to cancer drugs. Just 11% of treatments (mostly involving drugs) have been proven effective across some 3000 treatments examined by BMJ Clinical Evidence journal.
The media reported on October’s BMJ cancer drug study, but offered little in the way of opinion, certainly none that might cause people to rethink whether or not to try the latest cancer drug on offer. The only analysis that does the BMJ study justice, in our view, is one published recently by ANH friend and British medical journalist Jerome Burne.
He makes the case (abbreviated version below), that creating a truly sustainable healthcare system, will involve rethinking the way treatments are regulated and licensed ,a process that should involve non-pharmaceutical once as well. That’s something we’re considering in our own work that includes the development of criteria for more sustainable healthcare systems.
Over now to Jerome…
The following is an abbreviated version of Jerome’s article, ‘Most new cancer drugs won’t let you live longer or improve your quality of life’ that was published on HealthInsights UK on 13 October 2017. You can read the article in full via the link above.
Jerome Burne, UK medical journalist
“It is remarkable that so few cancer drugs enter the European market without any clear data on outcomes that matter to patients and their doctors: longer survival and better quality of life.” So goes the commentary by Huseyin Naci, Assistant Professor in the London School of Economic’s Department of Health Policy, co-author of the BMJ study.
These are the same medicines routinely marketed as ‘breakthrough drugs, yet the commentary describes them as giving the ‘false hope’ that they will work better than the older drugs they replace.
Ironically, giving ‘false hope’ is one of the most common charges laid against natural and non-drug ways of supporting cancer patients – yet the recent BMJ study found that, of the 68 cancer drugs approved by the European Medicines Agency (EMA) between 2009 and 2013, 57% were released onto the market without any clear evidence that they improved the quality or length of patients’ lives. The majority of them had been approved on ‘surrogate endpoints’, such as the cancerous tumour shrinking, which, the researchers made very clear, does not reliably predict you are going to live any longer.
Will Better Targeting of Genes Cure Cancer?
It’s a shameful finding, which should prompt patients and doctors alike to be sceptical of the latest ‘breakthrough’ drugs being rushed through the licensing process. Perhaps it may even lead to more serious attempts to test non-drug therapies such as the simple and non-invasive ketogenic diet.
The revelation comes at a time when cancer professionals are claiming that the long-promised transformation of cancer treatment by genetics is yet again just around the bend. However, there are serious reasons for believing that the limitations of the genetic approach mean that new, rushed gene targeting drugs are not going to effectively tackle cancer on their own. And if our current licencing system continues to allow a stream of useless drugs into the clinic, they won’t provide a cure however precisely they are targeted.
Not an Isolated Issue
The BMJ’s findings only relate to the European regulatory system , however this is far from being a Europe-only issue. A 2015 report in the Journal of the American Medical Association (JAMA) revealed that the American drug regulator – the FDA – was approving new cancer drugs on the basis of surrogate end points just as the EU was 2 years later. The authors concluded: “most cancer drug approvals have not been shown to, or do not, improve clinically relevant end points.”
Between 2008 and 2012, 38 cancer drugs were licensed, 67% on this basis. A check on survival rates four years later found that five of the drugs had improved life expectancy – but 18 had not. A deal with American companies whose drugs were nodded through relied on such efficacy trials being undertaken yet, four years later, a third of said companies had made zero effort towards running such tests.
It is clear that the interests of the supposedly most important people in this whole sorry saga – the patients – seem to have been totally ignored.
What does the Future Hold?
Everything points to the fact that we have a broken regulatory system. As the researchers of the BMJ so aptly stated, “when expensive drugs that lack clinically meaningful benefits are approved and reimbursed within publicly funded healthcare systems, individual patients may be harmed, important resources wasted, and the delivery of equitable and affordable care is undermined.”
With plans such as the 100,000 Genomes Project’s blindly optimistic reliance on the honour of companies running reliable clinical trials and follow-ups of new cancer drugs, it is hard not to see how the aforementioned ‘false hope’ is similarly embedded in the vision of gene-targeted treatments. To recognise that this current system is far from working is at least the first step in making treatment both safer and more effective for patients suffering from cancer. The idea that all orthodox drugs are scientifically based is a myth – and so is the idea that nothing other than gene-targeted drugs can ever be of use in treating cancer patients.
Listen to Jerome Burne discuss the ‘evidence of progress’ on UK Health Radio.
Acknowledgement Citation
Source and Further Information
Rob Verkerk | ANH-Intl <info@anhinternational.org>
Five Things You Should Know before Consenting to HPV Vaccination
http://anhinternational.org/2017/11/08/five-things-before-consenting-hpv-vaccine/
No parent wants their child to get cancer. And if there was a vaccine that could reduce the risk of certain kinds of cancers with a minimum of risk – most parents would opt for it with open arms.
Unfortunately, the 10-year track record of use of the vaccine, in varying forms, tells us that the claim being made by health authorities that the HPV vaccine is both ‘safe and effective’ is false.
In terms of claimed safety, health authorities are ignoring or seeking to cover-up the scale and severity of the serious and long-term side effects being experienced by adolescent girls. Regarding effectiveness, health authorities are misleading the public by claiming that the vaccine’s ability to raise HPV antibodies over a few years equates to protection from HPV-related cancers, especially cervical cancer, decades down the line. The science tells us that the two are not equivalent.
Five Things You Should Know Before Consenting to HPV vaccination
1. Be informed before giving consent! Health authorities responsible for national vaccination programs DO NOT offer informed consent. Proper informed consent is about giving a patient whatever information they need to make a decision about proposed medical treatment, including about non-vaccination based approaches. Not what a doctor or nurse thinks they should be told. This should include both risks and benefits, plus alternatives to said treatment. A landmark case on informed consent in the UK means that informed consent should include whatever a patient wants to know, not just what a doctor or nurse thinks they should be told.
To find out more, search ‘HPV vaccine’ on:
- ANH International HPV Vaccine Choice campaign
- ANH-USA Protect Vaccine Choice campaign
- European Medicines database on adverse drug reactions, EudraVigilance. Search for Gardasil, Gardasil 9 and Cervarix.
- Vaccine Adverse Event Reporting System (VAERS) US website. Search for Gardasil, Gardasil 9 and Cervarix.
- HPVvax Film website – soon to be released ANH-USA documentary website
- SaneVax
- National Vaccine Information Center
2. Health authorities are misleading the public when they imply that HPV vaccine confers protection against cervical cancer. It’s simply too early to tell how well elevated immunogenicity (i.e. being ‘seropositive’) prevents future cases of cervical cancer. For example, the UK NHS says “The HPV vaccine is effective at stopping girls getting the types of HPV that cause most cervical cancers” without saying that many girls already have HPV before becoming sexually active and most infections resolve on their own through the action of a healthy immune system. Some strains of HPV, like HPV-16, that are associated with cervical cancer, appear to have co-evolved alongside humans and are widely present in girls and women both with and without cervical cancer.
3. HPV is largely a transient infection and 90% of HPV infections resolve naturally within 2 years without vaccines or other medical interventions.
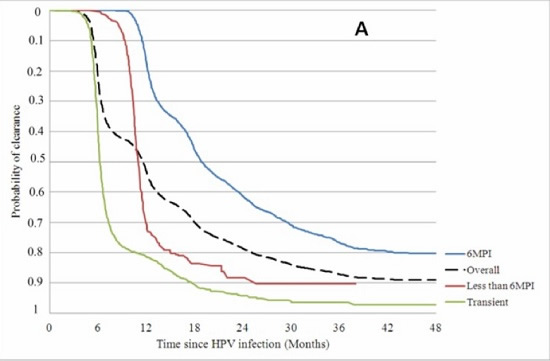
4. By far the majority (~80%) of cervical cancer incidence and deaths occur in developing countries. This appears to be associated with poor access to screening (Pap smear testing) or early treatment, higher rates of adolescent sexual activity, greater rates of STD infection, lower nutritional status, and other cervical cancer risk factors. Women in Western and industrialised countries are generally not told by health authorities that most of the cervical cancer burden occurs in developing countries.
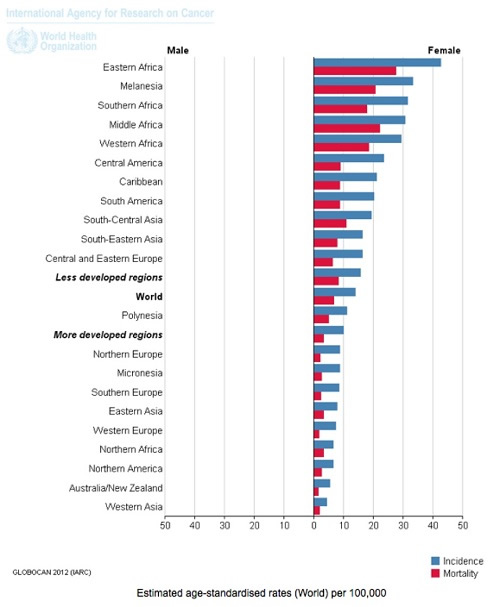
Cervical Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Source: IARC, WHO
5. Cervical cancer screening is crucial for early detection of cervical cancer. Women over 25 should be undergoing cervical cancer screening (Pap smear testing) every 3 to 5 years. HPV vaccine doesn’t eliminate the need for Pap smears that should occur more frequently in a woman’s 20s (every 3 years) than in later life (every five years). Owing to a risk of false negatives, as well as natural clearance of HPV or remission, annual testing should follow any detection of positive cytological results (cervical intra-epithelial neoplasia or CIN). Cancer treatment interventions should only be taken following positive results in more than two consecutive tests confirming early microinvasive cervical cancer that can be treated with a high degree of success using targeted techniques such as conisation with laser CO2 excision. Diet and lifestyle should also be modified to minimise future cancer risk.
Further Information
Reproduced from anhinternational.org - Five Things You Should Know before Consenting to HPV Vaccination.
Dr Yehuda Schoenfeld – Vaccines and Autoimmunity via J.B. Handley
https://www.facebook.com/jbhandleyjr/
Dr Yehuda Shoenfeld says vaccines cause auto-immunity. It's really not a question of "IF" there are adverse events from vaccines, it's a question of "how often?", "how severe?", and whether it's worth the trade-off? I recommend that you listen to the pre-eminent expert on vaccine-induced autoimmunity in the world.
https://m.youtube.com/watch?v=Wf8sw3n7xrE
This is just a clip from his talk, entire talk in comments below, as well as Dr Shoenfeld's new TEXTBOOK, called Vaccines and Autoimmunity! A List of all Vaccine Excipients has also been published
By the way, "autoimmunity" includes all the crazy epidemics in our kids that weren't around in the 1980s or earlier: asthma, food allergies, skin rashes, etc. "Some of the main examples of autoimmune disorders include diabetes mellitus type 1 (IDDM), systemic lupus erythematous (SLE), Hashimoto's thyroiditis, Graves' disease of the thyroid, Sjögren's syndrome, Churg-Strauss Syndrome, Coeliac disease, rheumatoid arthritis (RA), and idiopathic thrombocytopenic purport."
Listen to Dr Shoenfeld: "Dr Yehuda Shoenfeld is on the editorial board of 43 journals in the fields of rheumatology and autoimmunity and is the Founder and Editor of the Israel Medical Association Journal, the representative journal of science and medicine in the English language in Israel. He is also is the founder and editor of Autoimmunity Reviews and co-editor of The Journal of Autoimmunity. His clinical and scientific works focus on autoimmune and rheumatic diseases, and he has published more than 1700 papers in journals such as the New England Journal of Medicine, Nature, the Lancet, the Proceedings of the National Academy of Sciences of the United States of America, the Journal of Clinical Investigation, the Journal of Immunology, Blood, the Journal of the Federation of American Societies for Experimental Biology, the Journal of Experimental Medicine, Circulation, Cancer, and others, and his articles have had over 31,000 citations. He has written more than three hundred and fifty chapters in books, and has authored and edited 25 books."
Source and Further Information
https://www.facebook.com/jbhandleyjr/
https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
Children From Ghouta Telling Their Heart-breaking Story of Attack
I would like to bring to your attention the devastating situation currently unfolding in Eastern Ghouta, Syria. Though equal to the population and destruction of Aleppo before it’s fall, the situation has received very little media attention. I understand very few have the access to see this, but has the world given up on Syria?
This week, we have witnessed the most intense attacks Eastern Ghouta has ever seen. Heavy and continuous shelling, missiles, cluster bombs, napalm, chlorine gas and even prohibited incendiary weapons are being used in civilian areas. The use of toxic gases, such as chlorine, contravenes all accepted rules of war and is an abomination.
https://www.youtube.com/watch?v=QgTZTWW8Oao
As a father, it breaks my heart that 2 days ago a six-month-old infant died from congenital heart disease because doctors were not able to treat him or transport him to the care he needed 10 km away. The suffering of parents in Ghouta is unimaginable. Three infants died these past few weeks and thousands more are malnourished.
To make matters worse, there has been a wave of attacks on medical facilities. 3 medical facilities were attacked this week and 6 this month. Four ‘White Helmets’ rescue staff were killed on November 17th during a rescue. There have been over 600 people injured and 108 killed in the past week. Many of the medical facilities in Eastern Ghouta have been shut down and those remaining are overloaded with the influx of patients; the most they have seen in five years. The medical staff I have spoken to are physically and emotionally at their limits but have no other options. Even the most basic medicines/ medical supplies are in shortage with all aid supply lines cut off.
PEOPLE ARE STARVING! Can you imagine, the last shipment of food from the United Nations entered Eastern Ghouta at the end of last month. These supplies were barely sufficient to cover 10% of the population. Malnutrition and starvation are reaching epidemic levels.
The suffering of civilians in Eastern Ghouta is not a necessity of war, nor was it the only option. Despite the untold horrors, there are between 600-1,000 new births every month in Eastern Ghouta. The people of Ghouta still have hope for a brighter future and they deserve one.
What Can You Do:
- Join the global advocacy campaign on social media #BreakGhoutaSeige & #SaveEastGhouta ;
- Call, write and meet your local/ national political leaders. Urge them to take actions to end the siege and open safe aid corridors into Eastern Ghouta for: medical aid, food supplies and the immediate medical evacuation of 240 patients in critical condition;
- Organize events and hold vigils on behalf of the people of Eastern Ghouta;
- Write 'opinion editorials' to submit to your local media;
- Donate to our "Emergency Appeal: Eastern Ghouta Fund" to support medical aid inside Ghouta. https://uossmtr.nationbuilder.com/save_eastern_ghouta
Source and Further Information
Dr. Ghanem Tayara
Chairman, UOSSM International
https://uossmtr.nationbuilder.com/save_eastern_ghouta
https://www.youtube.com/watch?v=QgTZTWW8Oao
Media Inquiries:
Avi D'Souza - Global Director Of Communications, UOSSM International
Tel: (647) 528-5029
Comments:
-
No Article Comments available